miR-24 Inhibition Increases Menin Expression and Decreases Cholangiocarcinoma Proliferation
- PMID: 28087162
- PMCID: PMC5389363
- DOI: 10.1016/j.ajpath.2016.10.021
miR-24 Inhibition Increases Menin Expression and Decreases Cholangiocarcinoma Proliferation
Abstract
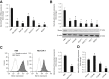
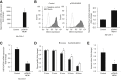
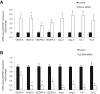
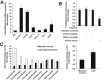
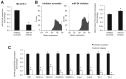
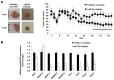
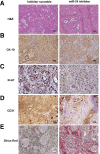
Menin (MEN1) is a tumor-suppressor protein in neuroendocrine tissue. Therefore, we tested the novel hypothesis that menin regulates cholangiocarcinoma proliferation. Menin and miR-24 expression levels were measured in the following intrahepatic and extrahepatic cholangiocarcinoma (CCA) cell lines, Mz-ChA-1, TFK-1, SG231, CCLP, HuCCT-1, and HuH-28, as well as the nonmalignant human intrahepatic biliary line, H69. miR-24 miRNA and menin protein levels were manipulated in vitro in Mz-ChA-1 cell lines. Markers of proliferation and angiogenesis (Ki-67, vascular endothelial growth factors A/C, vascular endothelial growth factor receptors 2/3, angiopoietin 1/2, and angiopoietin receptors 1/2) were evaluated. Mz-ChA-1 cells were injected into the flanks of nude mice and treated with miR-24 inhibitor or inhibitor scramble. Menin expression was decreased in advanced CCA specimens, whereas miR-24 expression was increased in CCA. Menin overexpression decreased proliferation, angiogenesis, migration, and invasion. Inhibition of miR-24 increased menin protein expression while decreasing proliferation, angiogenesis, migration, and invasion. miR-24 was shown to negatively regulate menin expression by luciferase assay. Tumor burden and expression of proliferative and angiogenic markers was decreased in the miR-24 inhibited tumor group compared to controls. Interestingly, treated tumors were more fibrotic than the control group. miR-24-dependent expression of menin may be important in the regulation of nonmalignant and CCA proliferation and may be an additional therapeutic tool for managing CCA progression.
Copyright © 2017 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Komuta M., Govaere O., Vandecaveye V., Akiba J., Van Steenbergen W., Verslype C., Laleman W., Pirenne J., Aerts R., Yano H., Nevens F., Topal B., Roskams T. Histological diversity in cholangiocellular carcinoma reflects the different cholangiocyte phenotypes. Hepatology. 2012;55:1876–1888. - PubMed
-
- Alvaro D., Mancino M.G., Glaser S., Gaudio E., Marzioni M., Francis H., Alpini G. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology. 2007;132:415–431. - PubMed
-
- Chandrasekharappa S.C., Guru S.C., Manickam P., Olufemi S.-E., Collins F.S., Emmert-Buck M.R., Debelenko L.V., Zhuang Z., Lubensky I.A., Liotta L.A., Crabtree J.S., Wang Y., Roe B.A., Weisemann J., Boguski M.S., Agarwal S.K., Kester M.B., Kim Y.S., Heppner C., Dong Q., Spiegel A.M., Burns A.L., Marx S.J. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997;276:404–407. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

