On-going Mechanical Damage from Mastication Drives Homeostatic Th17 Cell Responses at the Oral Barrier
- PMID: 28087239
- PMCID: PMC5263257
- DOI: 10.1016/j.immuni.2016.12.010
On-going Mechanical Damage from Mastication Drives Homeostatic Th17 Cell Responses at the Oral Barrier
Abstract
Immuno-surveillance networks operating at barrier sites are tuned by local tissue cues to ensure effective immunity. Site-specific commensal bacteria provide key signals ensuring host defense in the skin and gut. However, how the oral microbiome and tissue-specific signals balance immunity and regulation at the gingiva, a key oral barrier, remains minimally explored. In contrast to the skin and gut, we demonstrate that gingiva-resident T helper 17 (Th17) cells developed via a commensal colonization-independent mechanism. Accumulation of Th17 cells at the gingiva was driven in response to the physiological barrier damage that occurs during mastication. Physiological mechanical damage, via induction of interleukin 6 (IL-6) from epithelial cells, tailored effector T cell function, promoting increases in gingival Th17 cell numbers. These data highlight that diverse tissue-specific mechanisms govern education of Th17 cell responses and demonstrate that mechanical damage helps define the immune tone of this important oral barrier.
Keywords: IL-17; T cells; Th17 cells; barrier immunity; mucosal immunology; oral immunity; periodontitis.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
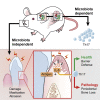
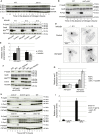
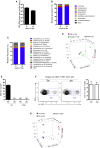
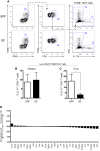

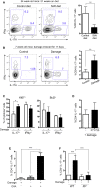
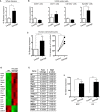
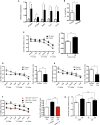
Comment in
-
Th17 Cells Require You to Chew before You Swallow.Immunity. 2017 Jan 17;46(1):8-10. doi: 10.1016/j.immuni.2016.12.016. Immunity. 2017. PMID: 28099867
-
T cells: Something to chew on.Nat Rev Immunol. 2017 Jan 31;17(2):81. doi: 10.1038/nri.2017.4. Nat Rev Immunol. 2017. PMID: 28138141 No abstract available.
References
-
- Atarashi K., Nishimura J., Shima T., Umesaki Y., Yamamoto M., Onoue M., Yagita H., Ishii N., Evans R., Honda K., Takeda K. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. - PubMed
-
- Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M., Weiner H.L., Kuchroo V.K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

