Myocardial relaxation is accelerated by fast stretch, not reduced afterload
- PMID: 28087265
- PMCID: PMC5347980
- DOI: 10.1016/j.yjmcc.2017.01.004
Myocardial relaxation is accelerated by fast stretch, not reduced afterload
Abstract
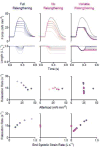
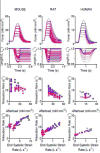
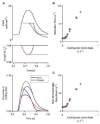
Fast relaxation of cross-bridge generated force in the myocardium facilitates efficient diastolic function. Recently published research studying mechanisms that modulate the relaxation rate has focused on molecular factors. Mechanical factors have received less attention since the 1980s when seminal work established the theory that reducing afterload accelerates the relaxation rate. Clinical trials using afterload reducing drugs, partially based on this theory, have thus far failed to improve outcomes for patients with diastolic dysfunction. Therefore, we reevaluated the protocols that suggest reducing afterload accelerates the relaxation rate and identified that myocardial relengthening was a potential confounding factor. We hypothesized that the speed of myocardial relengthening at end systole (end systolic strain rate), and not afterload, modulates relaxation rate and tested this hypothesis using electrically-stimulated trabeculae from mice, rats, and humans. We used load-clamp techniques to vary afterload and end systolic strain rate independently. Our data show that the rate of relaxation increases monotonically with end systolic strain rate but is not altered by afterload. Computer simulations mimic this behavior and suggest that fast relengthening quickens relaxation by accelerating the detachment of cross-bridges. The relationship between relaxation rate and strain rate is novel and upends the prevailing theory that afterload modifies relaxation. In conclusion, myocardial relaxation is mechanically modified by the rate of stretch at end systole. The rate of myocardial relengthening at end systole may be a new diagnostic indicator or target for treatment of diastolic dysfunction.
Keywords: Afterload; Cross-bridge; Diastole; Myocardium; Relaxation; Strain rate.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
None
Figures


References
-
- Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–50. - PubMed
-
- Babu GJ, Bhupathy P, Petrashevskaya NN, Wang H, Raman S, Wheeler D, et al. Targeted overexpression of sarcolipin in the mouse heart decreases sarcoplasmic reticulum calcium transport and cardiac contractility. J Biol Chem. 2006;281:3972–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

