Repair of 8-oxoG:A mismatches by the MUTYH glycosylase: Mechanism, metals and medicine
- PMID: 28087410
- PMCID: PMC5457711
- DOI: 10.1016/j.freeradbiomed.2017.01.008
Repair of 8-oxoG:A mismatches by the MUTYH glycosylase: Mechanism, metals and medicine
Abstract
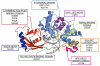
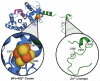
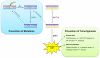
Reactive oxygen and nitrogen species (RONS) may infringe on the passing of pristine genetic information by inducing DNA inter- and intra-strand crosslinks, protein-DNA crosslinks, and chemical alterations to the sugar or base moieties of DNA. 8-Oxo-7,8-dihydroguanine (8-oxoG) is one of the most prevalent DNA lesions formed by RONS and is repaired through the base excision repair (BER) pathway involving the DNA repair glycosylases OGG1 and MUTYH in eukaryotes. MUTYH removes adenine (A) from 8-oxoG:A mispairs, thus mitigating the potential of G:C to T:A transversion mutations from occurring in the genome. The paramount role of MUTYH in guarding the genome is well established in the etiology of a colorectal cancer predisposition syndrome involving variants of MUTYH, referred to as MUTYH-associated polyposis (MAP). In this review, we highlight recent advances in understanding how MUTYH structure and related function participate in the manifestation of human disease such as MAP. Here we focus on the importance of MUTYH's metal cofactor sites, including a recently discovered "Zinc linchpin" motif, as well as updates to the catalytic mechanism. Finally, we touch on the insight gleaned from studies with MAP-associated MUTYH variants and recent advances in understanding the multifaceted roles of MUTYH in the cell, both in the prevention of mutagenesis and tumorigenesis.
Keywords: 8-oxoguanine; Base excision repair; Fe-S clusters; Glycosylase; MUTYH; MUTYH-associated polyposis; MutY.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures



References
-
- Su SS, Lahue RS, Au KG, Modrich P. Mispair specificity of methyl-directed DNA mismatch correction in vitro. The Journal of biological chemistry. 1988;263(14):6829–35. - PubMed
-
- David SS, Wiliams SD. Chemistry of glycosylases and endonucleases involved in base-excision repair. Chemical reviews. 1998;98(3):1221–1261. - PubMed
-
- Lindahl T. Uracil-DNA glycosylase from Escherichia coli. Methods Enzymol. 1980;65(1):284–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

