Wnt signaling balances specification of the cardiac and pharyngeal muscle fields
- PMID: 28087459
- PMCID: PMC5344754
- DOI: 10.1016/j.mod.2017.01.003
Wnt signaling balances specification of the cardiac and pharyngeal muscle fields
Abstract
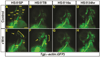
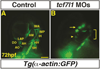
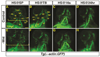
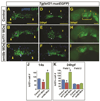
Canonical Wnt/β-catenin (Wnt) signaling plays multiple conserved roles during fate specification of cardiac progenitors in developing vertebrate embryos. Although lineage analysis in ascidians and mice has indicated there is a close relationship between the cardiac second heart field (SHF) and pharyngeal muscle (PM) progenitors, the signals underlying directional fate decisions of the cells within the cardio-pharyngeal muscle field in vertebrates are not yet understood. Here, we examined the temporal requirements of Wnt signaling in cardiac and PM development. In contrast to a previous report in chicken embryos that suggested Wnt inhibits PM development during somitogenesis, we find that in zebrafish embryos Wnt signaling is sufficient to repress PM development during anterior-posterior patterning. Importantly, the temporal sensitivity of dorso-anterior PMs to increased Wnt signaling largely overlaps with when Wnt signaling promotes specification of the adjacent cardiac progenitors. Furthermore, we find that excess early Wnt signaling can cell autonomously promote expansion of the first heart field (FHF) progenitors at the expense of PM and SHF within the anterior lateral plate mesoderm (ALPM). Our study provides insight into an antagonistic developmental mechanism that balances the sizes of the adjacent cardiac and PM progenitor fields in early vertebrate embryos.
Keywords: Cardiac; Mesoderm patterning; Organ fields; Pharyngeal muscle; Wnt signaling; Zebrafish.
Copyright © 2017 Elsevier B.V. All rights reserved.
Figures




References
-
- Baldini A. DiGeorge syndrome: the use of model organisms to dissect complex genetics. Hum Mol Genet. 2002;11:2363–2369. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

