Cathepsin K-targeted sub-micron particles for regenerative repair of vascular elastic matrix
- PMID: 28087488
- PMCID: PMC6361138
- DOI: 10.1016/j.actbio.2017.01.032
Cathepsin K-targeted sub-micron particles for regenerative repair of vascular elastic matrix
Abstract
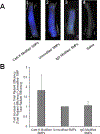
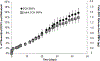
Abdominal Aortic Aneurysms (AAA) involve slow dilation and weakening of the aortic wall due to breakdown of structural matrix components, such as elastic fibers by chronically overexpressed matrix metalloproteinases (MMPs), primarily, MMPs-2 and -9. Auto-regenerative repair of disrupted elastic fibers by smooth muscle cells (SMCs) at the AAA site is intrinsically poor and together with chronic proteolysis prevents restoration of elastin homeostasis, necessary to enable AAA growth arrest or regression to a healthy state. Oral doxycycline (DOX) therapy can inhibit MMPs to slow AAA growth, but has systemwide side-effects and inhibits new elastin deposition within AAA tissue, diminishing prospects for restoring elastin homeostasis preventing the arrest/regression of AAA growth. We have thus developed cationic amphiphile (DMAB)-modified submicron particles (SMPs) that uniquely exhibit pro-elastogenic and anti-proteolytic properties, separate from similar effects of the encapsulated drug. These SMPs can enable sustained, low dose DOX delivery within AAA tissue to augment elastin regenerative repair. To provide greater specificity of SMP targeting, we have conjugated the DOX-SMP surface with an antibody against cathepsin K, a lysosomal protease that is highly overexpressed within AAA tissue. We have determined conditions for efficient cathepsin K Ab conjugation onto the SMPs, improved SMP binding to aneurysmal SMCs in culture and to injured vessel walls ex vivo, conjugation did not affect DOX release from the SMPs, and improved pro-elastogenic and anti-proteolytic effects due to the SMPs likely due to their increased proximity to cells via binding. Our study results suggest that cathepsin K Ab conjugation is a useful targeting modality for our pro-regenerative SMPs. Future studies will investigate SMP retention and biodistribution following targeting to induced AAAs in rat models through intravenous or catheter-based aortal infusion and thereafter their efficacy for regenerative elastic matrix repair in the AAA wall.
Statement of significance: Proactive screening of high risk elderly patients now enables early detection of Abdominal Aortic Aneurysms (AAAs). Current management of small, growing AAAs is limited to passive, imaging based growth monitoring. There are also no established drug-based therapeutic alternatives to surgery for AAAs, which is unsuitable for many elderly patients, and none which can achieve restore disrupted and lost elastic matrix in the AAA wall, which is essential to achieve growth arrest or regression. We seek to test the feasibility of a regenerative therapy based on localized, one time delivery of drug-releasing Sub-Micron-sized drug delivery polymer Particles (SMPs) that are also uniquely chemically functionalized on their surface to also provide them pro-elastin-regenerative & anti-matrix degradative properties, and also conjugated with antibodies targeting cathepsin K, an elastolytic enzyme that is highly overexpressed in AAA tissues; the latter serves as a modality to enable targeted binding of the SMPs to the AAA wall following intravenous infusion, or intraoartal, catheter-based delivery. Such SMPs can potentially stimulate structural repair in the AAA wall following one time infusion to delay or prevent AAA growth to rupture. The therapy can provide a non-surgical treatment option for high risk AAA patients.
Keywords: Cathepsin K; Drug delivery; Elastic matrix; Matrix metalloproteinases (MMPs); Regenerative matrix repair; Submicron particles.
Copyright © 2017 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures
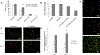



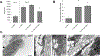

References
-
- Lederle FA, Johnson GR, Wilson SE, Chute EP, Littooy FN, Bandyk D, Krupski WC, Barone GW, Acher CW, Ballard DJ. Prevalence and associations of abdominal aortic aneurysm detection through screening. Ann Intern Med 1997;126;441–9. - PubMed
-
- Tanaka A, Hasegawa T, Chen Z, Okita Y, Okada K. A novel rat model of abdominal aortic aneurysm using a combination of intraluminal elastase infusion and extraluminal calcium chloride exposure. Journal for Vascular Surgery 2009;50;6;1423–32. - PubMed
-
- Upchurch GR Jr, Schaub TA. Abdominal Aortic Aneurysm. Am Fam Physician 2006;73;1198–204. - PubMed
-
- Bartoli MA, Parodi FE, Chu J, Pagano MB, Mao DL, Baxter BT, Buckley C, Ennis TL, Thompson RW. Localized administration of doxycycline suppresses aortic dilation in an experimental mouse model of abdominal aortic aneurysm. Ann Vasc Surg 2006;20;228–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

