Sympathetic modulation of electrical activation in normal and infarcted myocardium: implications for arrhythmogenesis
- PMID: 28087519
- PMCID: PMC5402014
- DOI: 10.1152/ajpheart.00575.2016
Sympathetic modulation of electrical activation in normal and infarcted myocardium: implications for arrhythmogenesis
Abstract
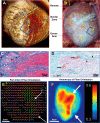
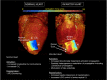
The influence of cardiac sympathetic innervation on electrical activation in normal and chronically infarcted ventricular myocardium is not understood. Yorkshire pigs with normal hearts (NL, n = 12) or anterior myocardial infarction (MI, n = 9) underwent high-resolution mapping of the anteroapical left ventricle at baseline and during left and right stellate ganglion stimulation (LSGS and RSGS, respectively). Conduction velocity (CV), activation times (ATs), and directionality of propagation were measured. Myocardial fiber orientation was determined using diffusion tensor imaging and histology. Longitudinal CV (CVL) was increased by RSGS (0.98 ± 0.11 vs. 1.2 ± 0.14m/s, P < 0.001) but not transverse CV (CVT). This increase was abrogated by β-adrenergic receptor and gap junction (GJ) blockade. Neither CVL nor CVT was increased by LSGS. In the peri-infarct region, both RSGS and LSGS shortened ARIs in sinus rhythm (423 ± 37 vs. 322 ± 30 ms, P < 0.001, and 423 ± 36 vs. 398 ± 36 ms, P = 0.035, respectively) and altered activation patterns in all animals. CV, as estimated by mean ATs, increased in a directionally dependent manner by RSGS (14.6 ± 1.2 vs. 17.3 ± 1.6 ms, P = 0.015), associated with GJ lateralization. RSGS and LSGS inhomogeneously modulated AT and induced relative or absolute functional activation delay in parts of the mapped regions in 75 and 67%, respectively, in MI animals, and in 0 and 15%, respectively, in control animals (P < 0.001 for both). In conclusion, sympathoexcitation increases CV in normal myocardium and modulates activation propagation in peri-infarcted ventricular myocardium. These data demonstrate functional control of arrhythmogenic peri-infarct substrates by sympathetic nerves and in part explain the temporal nature of arrhythmogenesis.NEW & NOTEWORTHY This study demonstrates regional control of conduction velocity in normal hearts by sympathetic nerves. In infarcted hearts, however, not only is modulation of propagation heterogeneous, some regions showed paradoxical conduction slowing. Sympathoexcitation altered propagation in all infarcted hearts studied, and we describe the temporal arrhythmogenic potential of these findings.Listen to this article's corresponding podcast at http://ajpheart.podbean.com/e/sympathetic-nerves-and-cardiac-propagation/.
Keywords: autonomic nervous system; conduction velocity; electrical propagation; sympathetic nerves; ventricular arrhythmias.
Copyright © 2017 the American Physiological Society.
Figures






Comment in
-
Ventricular tachycardia in ischemic heart disease: the sympathetic heart and its scars.Am J Physiol Heart Circ Physiol. 2017 Mar 1;312(3):H549-H551. doi: 10.1152/ajpheart.00061.2017. Epub 2017 Feb 10. Am J Physiol Heart Circ Physiol. 2017. PMID: 28188212 No abstract available.
References
-
- Ajijola OA, Yagishita D, Patel KJ, Vaseghi M, Zhou W, Yamakawa K, So E, Lux RL, Mahajan A, Shivkumar K. Focal myocardial infarction induces global remodeling of cardiac sympathetic innervation: neural remodeling in a spatial context. Am J Physiol Heart Circ Physiol 305: H1031–H1040, 2013. doi:10.1152/ajpheart.00434.2013. - DOI - PMC - PubMed
-
- Atkinson A, Inada S, Li J, Tellez JO, Yanni J, Sleiman R, Allah EA, Anderson RH, Zhang H, Boyett MR, Dobrzynski H. Anatomical and molecular mapping of the left and right ventricular His-Purkinje conduction networks. J Mol Cell Cardiol 51: 689–701, 2011. doi:10.1016/j.yjmcc.2011.05.020. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

