Molecular Pathways: Targeting Protein Tyrosine Phosphatases in Cancer
- PMID: 28087641
- PMCID: PMC5413367
- DOI: 10.1158/1078-0432.CCR-16-0934
Molecular Pathways: Targeting Protein Tyrosine Phosphatases in Cancer
Abstract
The aberrant activation of oncogenic signaling pathways is a universal phenomenon in cancer and drives tumorigenesis and malignant transformation. This abnormal activation of signaling pathways in cancer is due to the altered expression of protein kinases and phosphatases. In response to extracellular signals, protein kinases activate downstream signaling pathways through a series of protein phosphorylation events, ultimately producing a signal response. Protein tyrosine phosphatases (PTP) are a family of enzymes that hydrolytically remove phosphate groups from proteins. Initially, PTPs were shown to act as tumor suppressor genes by terminating signal responses through the dephosphorylation of oncogenic kinases. More recently, it has become clear that several PTPs overexpressed in human cancers do not suppress tumor growth; instead, they positively regulate signaling pathways and promote tumor development and progression. In this review, we discuss both types of PTPs: those that have tumor suppressor activities as well as those that act as oncogenes. We also discuss the potential of PTP inhibitors for cancer therapy. Clin Cancer Res; 23(9); 2136-42. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Figures
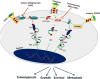
References
-
- Andersen JN, Jansen PG, Echwald SM, Mortensen OH, Fukada T, Del Vecchio R, et al. A genomic perspective on protein tyrosine phosphatases: gene structure, pseudogenes, and genetic disease linkage. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004;18(1):8–30. doi: 10.1096/fj.02-1212rev. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

