Novel Interconnections in Lipid Metabolism Revealed by Overexpression of Sphingomyelin Synthase-1
- PMID: 28087695
- PMCID: PMC5377821
- DOI: 10.1074/jbc.M116.751602
Novel Interconnections in Lipid Metabolism Revealed by Overexpression of Sphingomyelin Synthase-1
Abstract
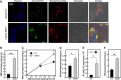
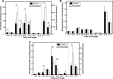
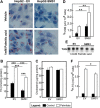
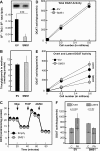
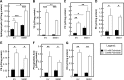
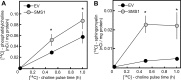
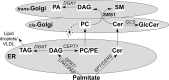
This study investigates the consequences of elevating sphingomyelin synthase 1 (SMS1) activity, which generates the main mammalian sphingolipid, sphingomyelin. HepG2 cells stably transfected with SMS1 (HepG2-SMS1) exhibit elevated enzyme activity in vitro and increased sphingomyelin content (mainly C22:0- and C24:0-sphingomyelin) but lower hexosylceramide (Hex-Cer) levels. HepG2-SMS1 cells have fewer triacylglycerols than controls but similar diacylglycerol acyltransferase activity, triacylglycerol secretion, and mitochondrial function. Treatment with 1 mm palmitate increases de novo ceramide synthesis in both cell lines to a similar degree, causing accumulation of C16:0-ceramide (and some C18:0-, C20:0-, and C22:0-ceramides) as well as C16:0- and C18:0-Hex-Cers. In these experiments, the palmitic acid is delivered as a complex with delipidated BSA (2:1, mol/mol) and does not induce significant lipotoxicity. Based on precursor labeling, the flux through SM synthase also increases, which is exacerbated in HepG2-SMS1 cells. In contrast, palmitate-induced lipid droplet formation is significantly reduced in HepG2-SMS1 cells. [14C]Choline and [3H]palmitate tracking shows that SMS1 overexpression apparently affects the partitioning of palmitate-enriched diacylglycerol between the phosphatidylcholine and triacylglycerol pathways, to the benefit of the former. Furthermore, triacylglycerols from HepG2-SMS1 cells are enriched in polyunsaturated fatty acids, which is indicative of active remodeling. Together, these results delineate novel metabolic interactions between glycerolipids and sphingolipids.
Keywords: ceramide; diacylglycerol; hepatocytes; lipid droplets; lipid metabolism; palmitic acid; phosphatidylcholine; phospholipid; sphingomyelin synthase; triacylglycerol.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
-
- Tafesse F. G., Huitema K., Hermansson M., van der Poel S., van den Dikkenberg J., Uphoff A., Somerharju P., and Holthuis J. C. (2007) Both sphingomyelin synthases SMS1 and SMS2 are required for sphingomyelin homeostasis and growth in human HeLa cells. J. Biol. Chem. 282, 17537–17547 - PubMed
-
- Futerman A. H., Stieger B., Hubbard A. L., and Pagano R. E. (1990) Sphingomyelin synthesis in rat liver occurs predominantly at the cis and medial cisternae of the Golgi apparatus. J. Biol. Chem. 265, 8650–8657 - PubMed
-
- Merrill A. H. Jr., and Wang E. (1986) Biosynthesis of long-chain (sphingoid) bases from serine by LM cells: evidence for introduction of the 4-trans-double bond after de novo biosynthesis of N-acylsphinganine(s). J. Biol. Chem. 261, 3764–3769 - PubMed
-
- Kennedy E. P. (1957) Metabolism of lipides. Annu. Rev. Biochem. 26, 119–148 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

