Two Small Molecules Restore Stability to a Subpopulation of the Cystic Fibrosis Transmembrane Conductance Regulator with the Predominant Disease-causing Mutation
- PMID: 28087700
- PMCID: PMC5339754
- DOI: 10.1074/jbc.M116.751537
Two Small Molecules Restore Stability to a Subpopulation of the Cystic Fibrosis Transmembrane Conductance Regulator with the Predominant Disease-causing Mutation
Abstract
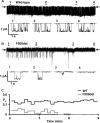
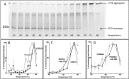
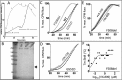
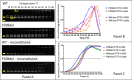
Cystic fibrosis (CF) is caused by mutations that disrupt the plasma membrane expression, stability, and function of the cystic fibrosis transmembrane conductance regulator (CFTR) Cl- channel. Two small molecules, the CFTR corrector lumacaftor and the potentiator ivacaftor, are now used clinically to treat CF, although some studies suggest that they have counteracting effects on CFTR stability. Here, we investigated the impact of these compounds on the instability of F508del-CFTR, the most common CF mutation. To study individual CFTR Cl- channels, we performed single-channel recording, whereas to assess entire CFTR populations, we used purified CFTR proteins and macroscopic CFTR Cl- currents. At 37 °C, low temperature-rescued F508del-CFTR more rapidly lost function in cell-free membrane patches and showed altered channel gating and current flow through open channels. Compared with purified wild-type CFTR, the full-length F508del-CFTR was about 10 °C less thermostable. Lumacaftor partially stabilized purified full-length F508del-CFTR and slightly delayed deactivation of individual F508del-CFTR Cl- channels. By contrast, ivacaftor further destabilized full-length F508del-CFTR and accelerated channel deactivation. Chronic (prolonged) co-incubation of F508del-CFTR-expressing cells with lumacaftor and ivacaftor deactivated macroscopic F508del-CFTR Cl- currents. However, at the single-channel level, chronic co-incubation greatly increased F508del-CFTR channel activity and temporal stability in most, but not all, cell-free membrane patches. We conclude that chronic lumacaftor and ivacaftor co-treatment restores stability in a small subpopulation of F508del-CFTR Cl- channels but that the majority remain destabilized. A fuller understanding of these effects and the characterization of the small F508del-CFTR subpopulation might be crucial for CF therapy development.
Keywords: ABC transporter; chloride channel; cystic fibrosis; cystic fibrosis transmembrane conductance regulator (CFTR); ivacaftor; lumacaftor; protein purification; protein stability.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
R. C. F. consulted for Vertex Pharmaceuticals in 2015 and 2016
Figures




References
-
- Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J.-L., Drumm M. L., Iannuzzi M. C., Collins F. S., and Tsui L.-C. (1989) Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245, 1066–1073 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

