Acetazolamide potentiates the afferent drive to prefrontal cortex in vivo
- PMID: 28087816
- PMCID: PMC5256155
- DOI: 10.14814/phy2.13066
Acetazolamide potentiates the afferent drive to prefrontal cortex in vivo
Abstract
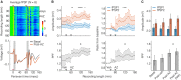
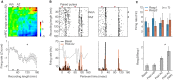
The knowledge on real-time neurophysiological effects of acetazolamide is still far behind the wide clinical use of this drug. Acetazolamide - a carbonic anhydrase inhibitor - has been shown to affect the neuromuscular transmission, implying a pH-mediated influence on the central synaptic transmission. To start filling such a gap, we chose a central substrate: hippocampal-prefrontal cortical projections; and a synaptic phenomenon: paired-pulse facilitation (a form of synaptic plasticity) to probe this drug's effects on interareal brain communication in chronically implanted rats. We observed that systemic acetazolamide potentiates the hippocampal-prefrontal paired-pulse facilitation. In addition to this field electrophysiology data, we found that acetazolamide exerts a net inhibitory effect on prefrontal cortical single-unit firing. We propose that systemic acetazolamide reduces the basal neuronal activity of the prefrontal cortex, whereas increasing the afferent drive it receives from the hippocampus. In addition to being relevant to the clinical and side effects of acetazolamide, these results suggest that exogenous pH regulation can have diverse impacts on afferent signaling across the neocortex.
Keywords: Carbonic anhydrase; hippocampus; prefrontal cortex; single‐unit activity; synaptic plasticity.
© 2017 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Figures

References
-
- Bartlett, E. L. , and Smith P. H.. 2002. Effects of paired‐pulse and repetitive stimulation on neurons in the rat medial geniculate body. Neuroscience 113:957–974. - PubMed
-
- Chesler, M. 2003. Regulation and modulation of pH in the brain. Physiol. Rev. 83:1183–1221. - PubMed
-
- Citri, A. , and Malenka R. C.. 2008. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology 33:18–41. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

