Factors associated with non-completion of follow-up: 33-month latent tuberculous infection treatment trial
- PMID: 28087928
- PMCID: PMC6563818
- DOI: 10.5588/ijtld.16.0469
Factors associated with non-completion of follow-up: 33-month latent tuberculous infection treatment trial
Abstract
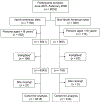
Setting: A post-hoc exploratory analysis of a randomized, open-label clinical trial that enrolled 8053 participants from the United States, Canada, Brazil, and Spain.
Objective: To assess factors associated with non-completion of study follow-up (NCF) in a 33-month latent tuberculous infection treatment trial, PREVENT TB.
Design: Participants were randomized to receive 3 months of weekly directly observed therapy vs. 9 months of daily self-administered therapy. NCF was defined as failing to be followed for at least 993 days (33 months) from enrollment. Possible factors associated with NCF were analyzed using univariate and multivariate regression via Cox proportional hazard model.
Results: Of 7061 adults selected for analysis, 841 (11.9%) did not complete study follow-up. Homelessness, young age, low education, history of incarceration, smoking, missing an early clinic visit, receiving isoniazid only, and male sex were significantly associated with NCF. Similar results were found in the North American region (United States and Canada) only. In Brazil and Spain, the only significant factor was missing an early clinic visit.
Conclusions: Study subjects at higher risk for NCF were identified by characteristics known at enrollment or in early follow-up. Evaluation of follow-up in other trials might help determine whether the identified factors consistently correlate with retention.
CONTEXTE:: Une analyse post hoc exploratoire d’un essai clinique randomisé ouvert qui a enrfôlé 8053 participants des Etats-Unis, du Canada, du Brésil et d’Espagne.
OBJECTIF:: Evaluer les facteurs associés au non achévement du suivi de l’étude (NCF) dans un essai thérapeutique de 33 mois de l’infection tuberculeuse latente, l’étude PREVENT TB.
SCHÉMA:: Les participants ont été randomisés pour recevoir 3 mois de traitement hebdomadaire sous observation directe contre 9 mois de traitement quotidien auto-administré. Le NCF a été défini comme un abandon du suivi, qui durait au moins 993 jours (33 mois) à partir de l’enrôlement. Les facteurs éventuels associés au NCF ont été; analysés par régression univariée et multivariée via le modéle de risque proportionnel de Cox.
RESULTATS:: Sur 7061 adultes sélectionnés pour l’analyse, 841 (11,9%) n’ont pas terminé le suivi de l’étude. Le fait d’être sans domicile fixe, le jeune âge, un faible niveau d’éducation, des antécedents d’incarcération, le tabagisme, une absence lors des premiéres consultations, avoir reçu de l’isoniazide seul et le sexe masculin ont été significativement associés au NCF. Des résultats similaires ont été constatés seulement en Amérique du Nord (Etats-Unis et Canada). Au Brésil et en Espagne, le seul facteur significatif a été l’absence à une des premierés consultations.
CONCLUSION:: Les sujets de l’étude à risque plus élevé de NCF ont été identifiés par des caractéristiques connues lors de l’enrôlement ou lors d’un suivi précoce. L’évaluation du suivi dans d’autres essais pourrait contribuer à déterminer si les facteurs identifiés sont corréles de maniére régulière avec la rétention.
MARCO DEREFERENCIA:: Un análisis a posteriori de un ensayo clínico aleatorizado sin anonimato, en el cual participaron 8053 personas de Estados Unidos, Canadá, Brasil y España.
OBJETIVO:: Evaluar los factores que se asocian con el incumplimiento de la integridad del seguimiento del estudio (NCF) en un ensayo clínico de 33 meses sobre el tratamiento de la infección tuberculosa latente (PREVENT TB).
MÉTODO:: Se randomizaron los participantes para recibir un tratamiento semanal directamente observado durante 3 meses o un tratamiento diario autoadministrado durante 9 meses. Se definió el NCF como la falta de un seguimiento mínimo de 993 días (33 meses) a partir de la inscripción en el estudio. Se examinaron los posibles factores asociados con el NCF mediante análisis de regresión univariante y multivariante, con un modelo de riesgos instantáneos proporcionales de Cox.
RESULTADOS:: De los 7061 adultos escogidos para el análisis, 841 no completaron el seguimiento del estudio (11,9%). Los factores que se asociaron de manera significativa con el NCF fueron la falta de domicilio, la juventud, el bajo grado de instrucción, el antecedente de encarcelamiento, el tabaquismo, la ausencia a una de las consultas iniciales, la administración exclusiva de isoniazida y el sexo masculino. Estos resultados se observaron solo en la región de Norteamérica (Estados Unidos y Canadá). En el Brasil y España, el único factor importante fue haber faltado a una de las consultas iniciales.
CONCLUSIÓN:: Fue posible reconocer a los participantes con riesgo de NCF a partir de características que se conocían en el momento de la inclusión en el estudio o durante la fase inicial del seguimiento. La evaluación del seguimiento de otros ensayos clínicos podría contribuir a determinar si los factores destacados en el presente estudio se correlacionan de manera constante con la permanencia en los estudios.
Trial registration: ClinicalTrials.gov NCT00023452.
Figures
References
-
- Conwell DS, Mosher A, Khan A, et al. Factors associated with loss to follow-up in a large tuberculosis treatment trial (TBTC Study 22). Contemp Clin Trials 2007; 28: 288–294. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

