Oral cannabidiol does not produce a signal for abuse liability in frequent marijuana smokers
- PMID: 28088032
- PMCID: PMC5361620
- DOI: 10.1016/j.drugalcdep.2016.11.030
Oral cannabidiol does not produce a signal for abuse liability in frequent marijuana smokers
Abstract
Background: Cannabidiol (CBD) is a naturally occurring constituent of the marijuana plant. In the past few years, there has been great interest in the therapeutic effects of isolated CBD and it is currently being explored for numerous disease conditions (e.g., pain, epilepsy, cancer, various drug dependencies). However, CBD remains a Schedule I drug on the U.S. Controlled Substances Act (CSA). Despite its status, there are no well-controlled data available regarding its abuse liability.
Methods: Healthy, frequent marijuana users (n=31) were enrolled in this within subject, randomized, placebo-controlled, double-blind, multisite study that administered oral cannabidiol (0, 200, 400, 800mg) alone and in combination with smoked marijuana (0.01%, 5.3-5.8% THC). Participants received one dose combination across 8 once-weekly outpatient sessions (7.5h). The primary findings on the drug interaction effects were previously reported (Haney et al., 2016). The present study is a secondary analysis of the data to examine the abuse liability profile of oral cannabidiol (200, 400, 800mg) in comparison to oral placebo and active smoked marijuana (5.3-5.8% THC).
Results: Active marijuana reliably produced abuse-related subjective effects (e.g., high) (p<0.05). However, CBD was placebo-like on all measures collected (p>0.05).
Conclusions: Overall, CBD did not display any signals of abuse liability at the doses tested and these data may help inform U.S. regulatory decisions regarding CBD schedule on the CSA.
Keywords: Abuse liability; CBD; Cannabidiol; Human; Smoked marijuana.
Copyright © 2016 Elsevier Ireland Ltd. All rights reserved.
Figures
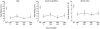
References
-
- Bergamaschi MM, Queiroz RH, Chagas MH, de Oliveira DC, De Martinis BS, Kapczinski F, Quevedo J, Roesler R, Schroder N, Nardi AE, Martin-Santos R, Hallak JE, Zuardi AW, Crippa JA. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naive social phobia patients. Neuropsychopharmacology. 2011;36:1219–1226. - PMC - PubMed
-
- Bhattacharyya S, Morrison PD, Fusar-Poli P, Martin-Santos R, Borgwardt S, Winton-Brown T, Nosarti C, CM OC, Seal M, Allen P, Mehta MA, Stone JM, Tunstall N, Giampietro V, Kapur S, Murray RM, Zuardi AW, Crippa JA, Atakan Z, McGuire PK. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35:764–774. - PMC - PubMed
-
- Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, Moriello AS, Davis JB, Mechoulam R, Di Marzo V. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134:845–852. - PMC - PubMed
-
- Consroe P, Laguna J, Allender J, Snider S, Stern L, Sandyk R, Kennedy K, Schram K. Controlled clinical trial of cannabidiol in Huntington's disease. Pharmacol Biochem Behav. 1991;40:701–708. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

