RhoA knockdown by cationic amphiphilic copolymer/siRhoA polyplexes enhances axonal regeneration in rat spinal cord injury model
- PMID: 28088077
- PMCID: PMC5315572
- DOI: 10.1016/j.biomaterials.2017.01.003
RhoA knockdown by cationic amphiphilic copolymer/siRhoA polyplexes enhances axonal regeneration in rat spinal cord injury model
Abstract
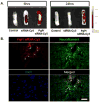
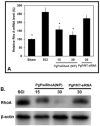
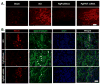
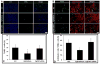
Spinal cord injury (SCI) results in permanent loss of motor and sensory function due to developmentally-related and injured-induced changes in the extrinsic microenvironment and intrinsic neuronal biochemistry that limit plasticity and axonal regeneration. Our long term goal is to develop cationic, amphiphilic copolymers (poly (lactide-co-glycolide)-g-polyethylenimine, PgP) for combinatorial delivery of therapeutic nucleic acids (TNAs) and drugs targeting these different barriers. In this study, we evaluated the ability of PgP to deliver siRNA targeting RhoA, a critical signaling pathway activated by multiple extracellular inhibitors of axonal regeneration. After generation of rat compression SCI model, PgP/siRhoA polyplexes were locally injected into the lesion site. Relative to untreated injury only, PgP/siRhoA polyplexes significantly reduced RhoA mRNA and protein expression for up to 4 weeks post-injury. Histological analysis at 4 weeks post-injury showed that RhoA knockdown was accompanied by reduced apoptosis, cavity size, and astrogliosis and increased axonal regeneration within the lesion site. These studies demonstrate that PgP is an efficient non-viral delivery carrier for therapeutic siRhoA to the injured spinal cord and may be a promising platform for the development of combinatorial TNA/drug therapy.
Keywords: Axon regeneration; Cationic amphiphilic co-polymer; Non-viral nucleic acid carrier; RhoA siRNA; Spinal cord injury.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures



References
-
- Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–6. - PubMed
-
- Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–9. - PubMed
-
- Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, et al. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417:941–4. - PubMed
-
- Liu BP, Fournier A, GrandPre T, Strittmatter SM. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297:1190–3. - PubMed
-
- Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz C, et al. PirB is a Functional Receptor for Myelin Inhibitors of Axonal Regeneration. Science. 2008;322:967–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

