Glycine-alanine dipeptide repeat protein contributes to toxicity in a zebrafish model of C9orf72 associated neurodegeneration
- PMID: 28088213
- PMCID: PMC5237533
- DOI: 10.1186/s13024-016-0146-8
Glycine-alanine dipeptide repeat protein contributes to toxicity in a zebrafish model of C9orf72 associated neurodegeneration
Abstract
Background: The most frequent genetic cause of frontotemporal lobar degeneration (FTLD) and amyotrophic lateral sclerosis (ALS) is the expansion of a GGGGCC hexanucleotide repeat in a non-coding region of the chromosome 9 open reading frame 72 (C9orf72) locus. The pathological hallmarks observed in C9orf72 repeat expansion carriers are the formation of RNA foci and deposition of dipeptide repeat (DPR) proteins derived from repeat associated non-ATG (RAN) translation. Currently, it is unclear whether formation of RNA foci, DPR translation products, or partial loss of C9orf72 predominantly drive neurotoxicity in vivo. By using a transgenic approach in zebrafish we address if the most frequently found DPR in human ALS/FTLD brain, the poly-Gly-Ala (poly-GA) protein, is toxic in vivo.
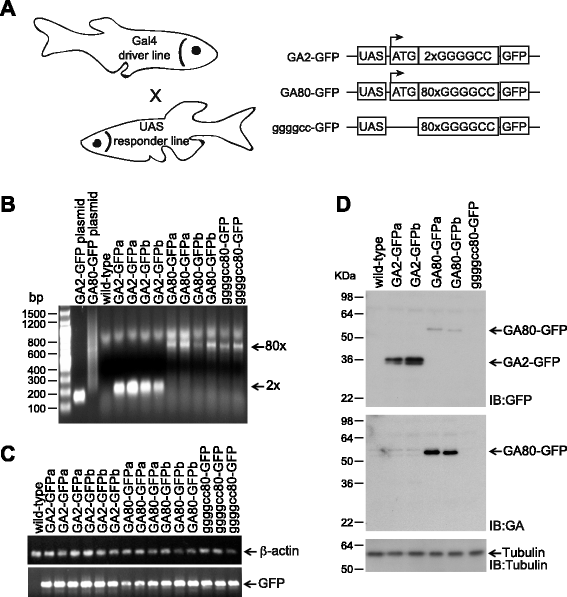
Method: We generated several transgenic UAS responder lines that express either 80 repeats of GGGGCC alone, or together with a translation initiation ATG codon forcing the translation of GA80-GFP protein upon crossing to a Gal4 driver. The GGGGCC repeat and GA80 were fused to green fluorescent protein (GFP) lacking a start codon to monitor protein translation by GFP fluorescence.
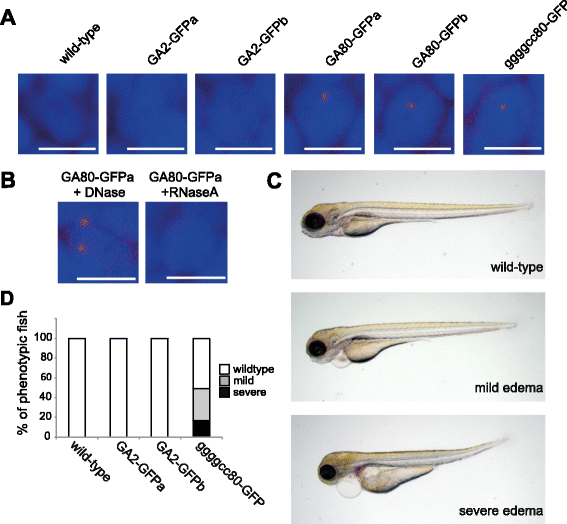
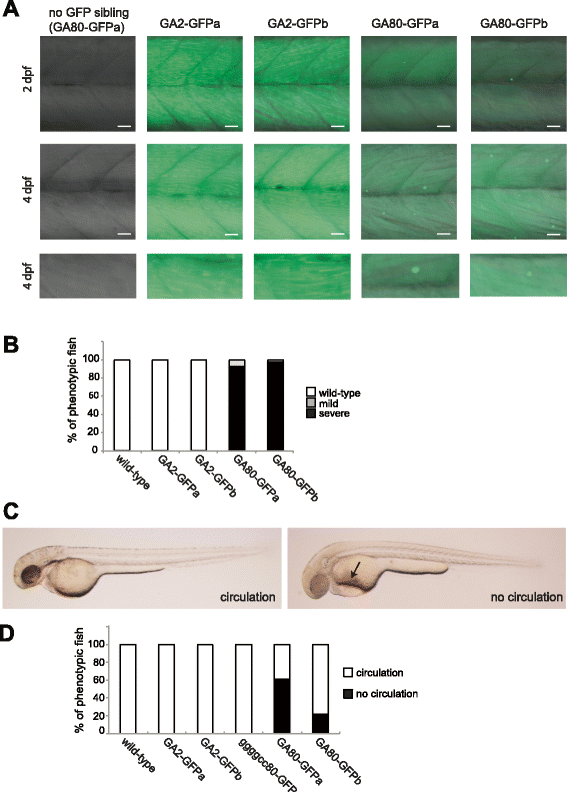
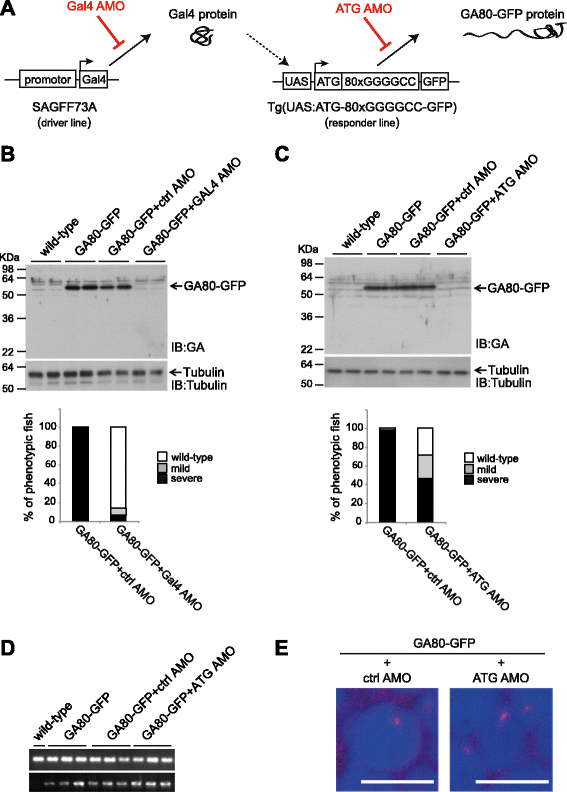
Results: Zebrafish transgenic for the GGGGCC repeat lacking an ATG codon showed very mild toxicity in the absence of poly-GA. However, strong toxicity was induced upon ATG initiated expression of poly-GA, which was rescued by injection of an antisense morpholino interfering with start codon dependent poly-GA translation. This morpholino only interferes with GA80-GFP translation without affecting repeat transcription, indicating that the toxicity is derived from GA80-GFP.
Conclusion: These novel transgenic C9orf72 associated repeat zebrafish models demonstrate poly-GA toxicity in zebrafish. Reduction of poly-GA protein rescues toxicity validating this therapeutic approach to treat C9orf72 repeat expansion carriers. These novel animal models provide a valuable tool for drug discovery to reduce DPR associated toxicity in ALS/FTLD patients with C9orf72 repeat expansions.
Keywords: C9orf72; Zebrafish; poly-GA toxicity.
Figures
Similar articles
-
C9orf72 FTLD/ALS-associated Gly-Ala dipeptide repeat proteins cause neuronal toxicity and Unc119 sequestration.Acta Neuropathol. 2014 Oct;128(4):485-503. doi: 10.1007/s00401-014-1329-4. Epub 2014 Aug 14. Acta Neuropathol. 2014. PMID: 25120191 Free PMC article.
-
eIF5 stimulates the CUG initiation of RAN translation of poly-GA dipeptide repeat protein (DPR) in C9orf72 FTLD/ALS.J Biol Chem. 2024 Mar;300(3):105703. doi: 10.1016/j.jbc.2024.105703. Epub 2024 Jan 30. J Biol Chem. 2024. PMID: 38301895 Free PMC article.
-
Bidirectional transcripts of the expanded C9orf72 hexanucleotide repeat are translated into aggregating dipeptide repeat proteins.Acta Neuropathol. 2013 Dec;126(6):881-93. doi: 10.1007/s00401-013-1189-3. Epub 2013 Oct 17. Acta Neuropathol. 2013. PMID: 24132570
-
Modelling C9ORF72 hexanucleotide repeat expansion in amyotrophic lateral sclerosis and frontotemporal dementia.Acta Neuropathol. 2014 Mar;127(3):377-89. doi: 10.1007/s00401-013-1235-1. Epub 2013 Dec 24. Acta Neuropathol. 2014. PMID: 24366528 Review.
-
Unconventional features of C9ORF72 expanded repeat in amyotrophic lateral sclerosis and frontotemporal lobar degeneration.Neurobiol Aging. 2014 Oct;35(10):2421.e1-2421.e12. doi: 10.1016/j.neurobiolaging.2014.04.015. Epub 2014 Apr 19. Neurobiol Aging. 2014. PMID: 24836899 Review.
Cited by
-
Molecular mechanisms underlying nucleotide repeat expansion disorders.Nat Rev Mol Cell Biol. 2021 Sep;22(9):589-607. doi: 10.1038/s41580-021-00382-6. Epub 2021 Jun 17. Nat Rev Mol Cell Biol. 2021. PMID: 34140671 Free PMC article. Review.
-
Zebrafish, Medaka and Turquoise Killifish for Understanding Human Neurodegenerative/Neurodevelopmental Disorders.Int J Mol Sci. 2022 Jan 26;23(3):1399. doi: 10.3390/ijms23031399. Int J Mol Sci. 2022. PMID: 35163337 Free PMC article. Review.
-
The neurological toxicity of heavy metals: A fish perspective.Comp Biochem Physiol C Toxicol Pharmacol. 2018 Jun;208:12-19. doi: 10.1016/j.cbpc.2017.11.008. Epub 2017 Dec 1. Comp Biochem Physiol C Toxicol Pharmacol. 2018. PMID: 29199130 Free PMC article. Review.
-
Stable transgenic C9orf72 zebrafish model key aspects of the ALS/FTD phenotype and reveal novel pathological features.Acta Neuropathol Commun. 2018 Nov 19;6(1):125. doi: 10.1186/s40478-018-0629-7. Acta Neuropathol Commun. 2018. PMID: 30454072 Free PMC article.
-
Zebrafish as a model organism for neurodegenerative disease.Front Mol Neurosci. 2022 Oct 13;15:940484. doi: 10.3389/fnmol.2022.940484. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36311026 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

