Spinal versus general anaesthesia in surgery for inguinodynia (SPINASIA trial): study protocol for a randomised controlled trial
- PMID: 28088218
- PMCID: PMC5237574
- DOI: 10.1186/s13063-016-1746-x
Spinal versus general anaesthesia in surgery for inguinodynia (SPINASIA trial): study protocol for a randomised controlled trial
Abstract
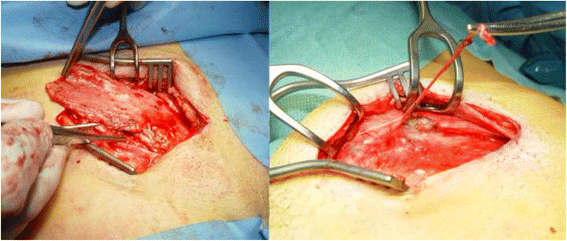
Background: Chronic inguinodynia (groin pain) is a common complication following open inguinal hernia repair or a Pfannenstiel incision but may also be experienced after other types of (groin) surgery. If conservative treatments are to no avail, tailored remedial surgery, including a neurectomy and/or a (partial) meshectomy, may be considered. Retrospective studies in patients with chronic inguinodynia suggested that spinal anaesthesia is superior compared to general anaesthesia in terms of pain relief following remedial operations. This randomised controlled trial is designed to study the effect of type of anaesthesia (spinal or general) on pain relief following remedial surgery for inguinodynia.
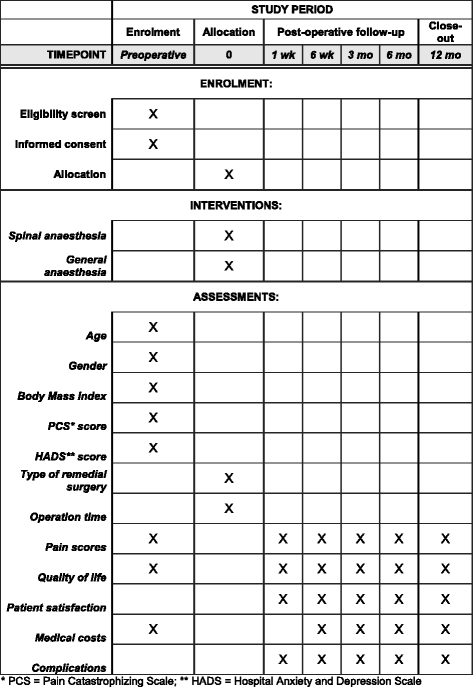
Methods: A total of 190 adult patients who suffer from unacceptable chronic (more than 3 months) inguinodynia, as subjectively judged by the patients themselves, are included. Only patients scheduled to undergo a neurectomy and/or a meshectomy by an open approach are considered for inclusion and randomised to spinal or general anaesthesia. Patients are excluded if pain is attributable to abdominal causes or if any contraindications for either type of anaesthesia are present. Primary outcome is effect of type of anaesthesia on pain relief. Secondary outcomes include patient satisfaction, quality of life, use of analgesics and (in)direct medical costs. Patient follow-up period is one year.
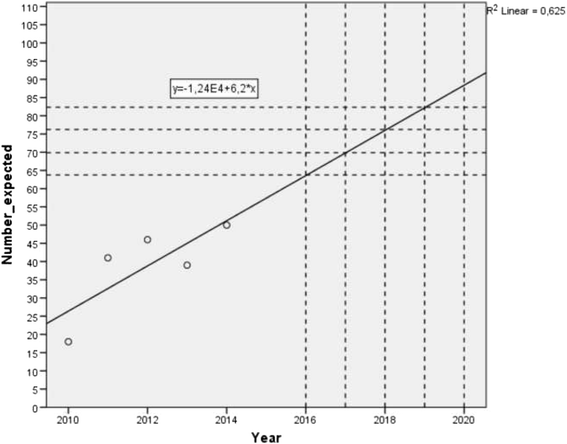
Discussion: The first patient was included in January 2016. The expected trial deadline is December 2019. Potential effects are deemed related to the entire setting of type of anaesthesia. Since any setting is multifactorial, all of these factors may influence the outcome measures. This is the first large randomised controlled trial comparing the two most frequently used anaesthetic techniques in remedial surgery for groin pain. There is a definite need for evidence-based strategies to optimise results of these types of surgery. Besides pain relief, other important patient-related outcome measures are assessed to include patient's perspectives on outcome.
Trial registration: The protocol (protocol number NL54115.015.15 ) is approved by the Medical Ethics Committee of Máxima Medical Centre, Veldhoven, The Netherlands. The study protocol was registered at www.trialregister.nl (NTR registration number: 5586) on 15 January 2016.
Keywords: Anesthesia; Chronic pain; Operative; Pain; Pain management; Postoperative; Randomised controlled trail [publication type]; Surgical procedures.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

