Ruxolitinib for symptom control in patients with chronic lymphocytic leukaemia: a single-group, phase 2 trial
- PMID: 28089238
- PMCID: PMC5356368
- DOI: 10.1016/S2352-3026(16)30194-6
Ruxolitinib for symptom control in patients with chronic lymphocytic leukaemia: a single-group, phase 2 trial
Abstract
Background: Disease-related symptoms impair the quality of life of patients with chronic lymphocytic leukaemia (CLL) who do not require systemic therapy. Available therapies are not specifically aimed at symptom control. Because stimulation of the B-cell receptor activates JAK2 in CLL cells and the JAK2 inhibitor ruxolitinib improves symptoms in patients with myelofibrosis, we postulated that ruxolitinib would improve disease-related symptoms in patients with CLL. We did a phase 2 trial of ruxolitinib to test this hypothesis.
Methods: Symptomatic patients with CLL who did not require systemic therapy were enrolled at MD Anderson Cancer Center (Houston, TX, USA) between Sept 15, 2014, and Sept 20, 2015. Participants were given 10 mg ruxolitinib orally twice a day. Scores on the Brief Fatigue Inventory (BFI), CLL module of the MD Anderson Symptom Inventory (MDASI) and symptom-associated interference in daily activities, were assessed before treatment and after 3 months. This trial is ongoing and is registered at ClinicalTrials.gov (NCT02131584).
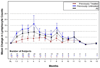
Findings: 41 patients (25 previously untreated for CLL and 16 previously treated) were enrolled. At 3 months, the mean percentage change from baseline in BFI score was 44·3% (SD 35·0, p<0·0001), in symptom interference score was 43·4% (51·5, p<0·0001), and in MDASI score was 42·1% (37·4, p<0·0001). 32 (78%) of the patients experienced 20% or greater reduction in the mean BFI, and 24 (59%) had a reduction of two units or more in worst fatigue score in past 24 hours as assessed by the BFI. The most comment grade 3-4 adverse events were neutropenia (n=2 [5%]), hypertension (n=2 [5%]), insomnia (n=1 [2%]), tinnitus and dizziness (n=1 [2%]), and thrombocytopenia (n=1 [2%]).
Interpretation: In patients with CLL, ruxolitinib was associated with significant improvements in disease-related symptoms as measured by BFI, MDASI, and symptom interference scores. Further studies to test the therapeutic efficacy of ruxolitinib in CLL are warranted.
Funding: Incyte, National Cancer Institute.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures

References
-
- Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456. - PMC - PubMed
-
- Efficace F, Kemmler G, Vignetti M, Mandelli F, Molica S, Holzner B. Health-related quality of life assessment and reported outcomes in leukaemia randomised controlled trials - a systematic review to evaluate the added value in supporting clinical decision making. Eur J Cancer. 2008;44(11):1497–1506. - PubMed
-
- Pashos CL, Flowers CR, Kay NE, et al. Association of health-related quality of life with gender in patients with B-cell chronic lymphocytic leukemia. Support Care Cancer. 2013;21(10):2853–2860. - PubMed
-
- Stephens JM, Gramegna P, Laskin B, Botteman MF, Pashos CL. Chronic lymphocytic leukemia: economic burden and quality of life: literature review. Am J Ther. 2005;12(5):460–466. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

