Efficacy and Toxicity of Intravitreous Chemotherapy for Retinoblastoma: Four-Year Experience
- PMID: 28089679
- PMCID: PMC5441308
- DOI: 10.1016/j.ophtha.2016.12.015
Efficacy and Toxicity of Intravitreous Chemotherapy for Retinoblastoma: Four-Year Experience
Abstract
Purpose: To investigate the efficacy and toxicity of intravitreous melphalan for treatment of retinoblastoma, as a single agent or with concomitant topotecan.
Participants: A total of 130 eyes of 120 patients with retinoblastoma receiving 630 intravitreous (melphalan, topotecan) or topotecan periocular injections. A total of 83 (64%) of these eyes were treated with concomitant ophthalmic artery chemosurgery (OAC).
Design: Retrospective cohort study.
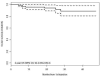
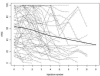
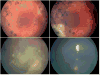
Methods: Indirect ophthalmoscopy and clinical imaging were used to evaluate clinical response. Ocular survival and disease-free survival were estimated using Kaplan-Meier methods in 130 eyes. Ocular toxicity was evaluated by clinical findings and electroretinography (ERG) on 244 evaluable injections in 63 patients using 30-Hz flicker responses. Analysis was performed using linear mixed effects models with a random intercept and slope for each patient and a fixed effect for number of injections, in addition to any other fixed effect of interest.
Main outcome measures: Ocular survival, disease-free survival, ERG: peak-to-peak ERG amplitudes in response to 30-Hz photopic flicker stimulation.
Results: There were no disease- or treatment-related deaths, and no patient developed externalization of tumor or metastatic disease. Two-year Kaplan-Meier estimates of ocular survival and disease-free survival were 94.2% (95% confidence interval, 89.2-99.4) and 86.2% (95% confidence interval, 78.7-94.5), respectively. There was a significant association between the number of injections and diminished ERG responses, such that on average each intravitreous melphalan injection was associated with a 5.3-μV decrease in ERG amplitude (P < 0.001). Concomitant intra-arterial chemotherapy (P = 0.01) and greater inherent ocular pigment also were significantly associated with a reduction in ERG (P = 0.045). Patient age and weight, new injection site location, addition of topotecan, concomitant focal treatment, and time interval between injections were not significantly associated with toxicity.
Conclusions: Intravitreous melphalan is an effective treatment for vitreous seeding in retinoblastoma, resulting in high rates of ocular survival and disease-free survival. However, in this study, each injection of melphalan was associated, on average, with a decrement in ERG response. The findings suggest increased toxicity (1) when OAC is given within 1 week of the intravitreous injection and (2) in more deeply pigmented eyes.
Copyright © 2017 American Academy of Ophthalmology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
No conflicting relationship exists for any author, except Marr B who is a consultatnt for Aura Biosciences, Inc.
Figures


References
-
- Munier FL, Gaillard MC, Balmer A, et al. Intravitreal chemotherapy for vitreous disease in retinoblastoma revisited: from prohibition to conditional indications. Br J Ophthalmol. 2012 - PubMed
-
- Francis JH, Schaiquevich P, Buitrago E, et al. Local and systemic toxicity of intravitreal melphalan for vitreous seeding in retinoblastoma: a preclinical and clinical study. Ophthalmology. 2014;121:1810–1817. - PubMed
-
- Abramson DH, Marr BP, Dunkel IJ, et al. Intra-arterial chemotherapy for retinoblastoma in eyes with vitreous and/or subretinal seeding: 2-year results. Br J Ophthalmol. 2012;96:499–502. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

