Co-feeding transmission facilitates strain coexistence in Borrelia burgdorferi, the Lyme disease agent
- PMID: 28089780
- PMCID: PMC5474356
- DOI: 10.1016/j.epidem.2016.12.002
Co-feeding transmission facilitates strain coexistence in Borrelia burgdorferi, the Lyme disease agent
Abstract
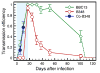
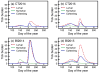
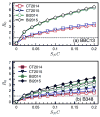
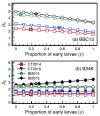
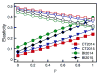
Coexistence of multiple tick-borne pathogens or strains is common in natural hosts and can be facilitated by resource partitioning of the host species, within-host localization, or by different transmission pathways. Most vector-borne pathogens are transmitted horizontally via systemic host infection, but transmission may occur in the absence of systemic infection between two vectors feeding in close proximity, enabling pathogens to minimize competition and escape the host immune response. In a laboratory study, we demonstrated that co-feeding transmission can occur for a rapidly-cleared strain of Borrelia burgdorferi, the Lyme disease agent, between two stages of the tick vector Ixodes scapularis while feeding on their dominant host, Peromyscus leucopus. In contrast, infections rapidly became systemic for the persistently infecting strain. In a field study, we assessed opportunities for co-feeding transmission by measuring co-occurrence of two tick stages on ears of small mammals over two years at multiple sites. Finally, in a modeling study, we assessed the importance of co-feeding on R0, the basic reproductive number. The model indicated that co-feeding increases the fitness of rapidly-cleared strains in regions with synchronous immature tick feeding. Our results are consistent with increased diversity of B. burgdorferi in areas of higher synchrony in immature feeding - such as the midwestern United States. A higher relative proportion of rapidly-cleared strains, which are less human pathogenic, would also explain lower Lyme disease incidence in this region. Finally, if co-feeding transmission also occurs on refractory hosts, it may facilitate the emergence and persistence of new pathogens with a more limited host range.
Keywords: Borrelia; Co-feeding; Coexistence; Ixodes scapularis; Lyme disease; Pathogen diversity.
Copyright © 2016 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
We have no competing interests.
Figures

References
-
- Andersson M, Scherman K, Raberg L. Multiple-strain infections of Borrelia afzelii: a role for within-host interactions in the maintenance of antigenic diversity? Am Nat. 2013;181(4):545–554. - PubMed
-
- Brunner JL, Ostfeld RS. Multiple causes of variable tick burdens on small-mammal hosts. Ecology. 2008;89(8):2259–2272. - PubMed
-
- Crippa M, Rais O, Gern L. Investigations on the mode and dynamics of transmission and infectivity of Borrelia burgdorferi sensu stricto and Borrelia afzelii in Ixodes ricinus ticks. Vector Borne Zoonotic Dis. 2002;2(1):3–9. - PubMed
-
- Davis S, Bent SJ. Loop analysis for pathogens: niche partitioning in the transmission graph for pathogens of the North American tick Ixodes scapularis. J Theor Biol. 2011;269(1):96–103. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

