Novel methods in pulmonary hypertension phenotyping in the age of precision medicine (2015 Grover Conference series)
- PMID: 28090286
- PMCID: PMC5210071
- DOI: 10.1086/688847
Novel methods in pulmonary hypertension phenotyping in the age of precision medicine (2015 Grover Conference series)
Erratum in
-
Corrigendum.Pulm Circ. 2017 Apr-Jun;7(2):559. doi: 10.1177/2045893217706334. Pulm Circ. 2017. PMID: 28597768 Free PMC article. No abstract available.
Abstract
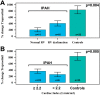
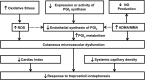
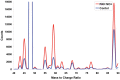
Among pulmonary vascular diseases, pulmonary hypertension (PH) is the best studied and has been the focus of our work. The current classification of PH is based on a relatively simple combination of patient characteristics and hemodynamics. This leads to inherent limitations, including the inability to customize treatment and the lack of clarity from a more granular identification based on individual patient phenotypes. Accurate phenotyping of PH can be used in the clinic to select therapies and determine prognosis and in research to increase the homogeneity of study cohorts. Rapid advances in the mechanistic understanding of the disease, improved imaging methods, and innovative biomarkers now provide an opportunity to define novel PH phenotypes. We have recently shown that altered metabolism may affect nitric oxide levels and protein glycosylation, the peripheral circulation (which may provide insights into the response to therapy), and exhaled-breath analysis (which may be useful in disease evaluation). This review is based on a talk presented during the 2015 Grover Conference and highlights the relevant literature describing novel methods to phenotype pulmonary arterial hypertension patients by using approaches that involve the pulmonary and systemic (peripheral) vasculature. In particular, abnormalities in metabolism, the pulmonary and peripheral circulation, and exhaled breath in PH may help identify phenotypes that can be the basis for a precision-medicine approach to PH management. These approaches may also have a broader scope and may contribute to a better understanding of other diseases, such as asthma, diabetes, and cancer.
Keywords: exhaled breath; metabolism; peripheral circulation; phenotyping; precision medicine.
Figures


References
-
- Butrous G, Ghofrani HA, Grimminger F. Pulmonary vascular disease in the developing world. Circulation 2008;118(17):1758–1766. - PubMed
-
- Koress C, Swan K, Kadowitz P. Soluble guanylate cyclase stimulators and activators: novel therapies for pulmonary vascular disease or a different method of increasing cGMP? Curr Hypertens Rep 2016;18(5):42. doi:10.1007/s11906-016-0645-6. - PubMed
-
- Barnett CF, Alvarez P, Park MH. Pulmonary arterial hypertension: diagnosis and treatment. Cardiol Clin 2016;34(3):375–389. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

