Chronic mucocutaneous candidiasis disease associated with inborn errors of IL-17 immunity
- PMID: 28090315
- PMCID: PMC5192062
- DOI: 10.1038/cti.2016.71
Chronic mucocutaneous candidiasis disease associated with inborn errors of IL-17 immunity
Abstract
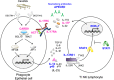
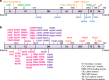
Chronic mucocutaneous candidiasis (CMC) is characterized by recurrent or persistent infections affecting the nails, skin and oral and genital mucosae caused by Candida spp., mainly Candida albicans. CMC is an infectious phenotype in patients with inherited or acquired T-cell deficiency. Patients with autosomal-dominant (AD) hyper IgE syndrome (HIES), AD signal transducer and activator of transcription 1 (STAT1) gain-of-function, autosomal-recessive (AR) deficiencies in interleukin (IL)-12 receptor β1 (IL-12Rβ1), IL-12p40, caspase recruitment domain-containing protein 9 (CARD9) or retinoic acid-related orphan receptor γT (RORγT) or AR autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) develop CMC as a major infectious phenotype that is categorized as Syndromic CMC. In contrast, CMC disease (CMCD) is typically defined as CMC in patients in the absence of any other prominent clinical signs. This definition is not strict; thus, CMCD is currently used to refer to patients presenting with CMC as the main clinical phenotype. The etiology of CMCD is not related to genes that cause severe combined immunodeficiency or combined immunodeficiency, nor to genes responsible for Syndromic CMC. Four genetic etiologies, AR IL-17 receptor A, IL-17 receptor C and ACT1 deficiencies, and AD IL-17F deficiency, are reported to underlie CMCD. Each of these gene defects directly has an impact on IL-17 signaling, suggesting their nonredundant role in host mucosal immunity to Candida. Here, we review current knowledge focusing on IL-17 signaling and the genetic etiologies responsible for, and associated with, CMC.
Figures

References
-
- Kirkpatrick CH. Chronic mucocutaneous candidiasis. Pediatr Infect Dis J 2001; 20: 197–206. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
