Vaccination to gain humoral immune memory
- PMID: 28090322
- PMCID: PMC5192068
- DOI: 10.1038/cti.2016.81
Vaccination to gain humoral immune memory
Abstract
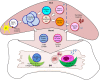
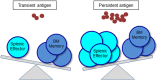
The concept of immune memory forms the biological basis for vaccination programs. Despite advancements in the field of immune memory and vaccination, most current vaccines are evaluated by magnitude of antigen-specific antibody titers in serum or mucosa after vaccination. It has been shown, however, that antibody-mediated humoral immune memory is established regardless of the magnitude and duration of immune reactions, suggesting that assessment of vaccine efficacy should be performed for several years after vaccination. This long-term investigation is disadvantageous for prevalent and pandemic infections. Long-lived memory plasma cells and memory helper T cells which contribute to humoral immune memory are generated in the bone marrow after migration of memory cell precursors through bloodstream. Thus, it may be a novel evaluation strategy to assess the precursors of memory cells in the blood in the early phase of the immune reaction(s). We here review recent advances on the generation and maintenance of immune memory cells involved in humoral immunity and introduce a current concept of direct and short-term assessment of humoral immune memory formation upon vaccination as a correlate of protection.
Figures
References
-
- Fenner F. A successful eradication campaign. Global eradication of smallpox. Rev Infect Dis 1982; 4: 916–930. - PubMed
-
- Willis NJ. Edward Jenner and the eradication of smallpox. Scott Med J 1997; 42: 118–121. - PubMed
-
- Rappuoli R, Miller HI, Falkow S. Medicine. The intangible value of vaccination. Science 2002; 297: 937–939. - PubMed
-
- Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis 2008; 47: 401–409. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
