Top-down Infliximab Study in Kids with Crohn's disease (TISKids): an international multicentre randomised controlled trial
- PMID: 28090335
- PMCID: PMC5223648
- DOI: 10.1136/bmjgast-2016-000123
Top-down Infliximab Study in Kids with Crohn's disease (TISKids): an international multicentre randomised controlled trial
Abstract
Introduction: Crohn's disease (CD) is a chronic inflammatory disease predominantly affecting the gastrointestinal tract. CD usually requires lifelong medication and is accompanied by severe complications, such as fistulae and strictures, resulting in surgery. Infliximab (IFX) is very effective for treating paediatric patients with CD, but is currently only registered for therapy refractory patients-the so-called step-up strategy. We hypothesise that using IFX first-line, that is, top-down, will give more mucosal healing, fewer relapses, less complications, need for surgery and hospitalisation.
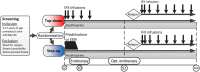
Methods and analysis: This international multicentre open-label randomised controlled trial includes children, aged 3-17 years, with new-onset, untreated CD with moderate-to-severe disease activity (weighted Paediatric Crohn's Disease Activity Index (wPCDAI)>40). Eligible patients will be randomised to top-down or step-up treatment. Top-down treatment consists of 5 IFX infusions combined with azathioprine (AZA). After these 5 infusions, patients will continue AZA. Patients randomised to step-up will receive standard induction treatment, either oral prednisolone or exclusive enteral nutrition, combined with AZA as maintenance treatment. The primary outcome is clinical remission (wPCDAI<12.5) at 52 weeks without need for additional CD-related therapy or surgery. Total follow-up is 5 years. Secondary outcomes include clinical disease activity, mucosal healing by endoscopy (at week 10 and optionally week 52), faecal calprotectin, growth, quality of life, medication use and adverse events.
Ethics and dissemination: Conducted according to the Declaration of Helsinki and Good Clinical Practice. Medical-ethical approval will be obtained for each site.
Trial registration number: NCT02517684; Pre-results.
Keywords: COST-EFFECTIVENESS; CROHN'S DISEASE; INFLIXIMAB; PAEDIATRIC GASTROENTEROLOGY; STEROID-SPARING EFFICACY.
Conflict of interest statement
JCE is part of the Scientific Advisory Committee of Janssen and Abbvie and received a research grant from Merck Sharp & Dohme (MSD). LdR reports a presentation on an Abbvie sponsored meeting.
Figures
References
-
- Levine A, Koletzko S, Turner D, et al. . ESPGHAN revised Porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr 2014;58:795–806. doi:10.1097/MPG.0000000000000239 - DOI - PubMed
-
- Benchimol EI, Fortinsky KJ, Gozdyra P, et al. . Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis 2011;17:423–39. doi:10.1002/ibd.21349 - DOI - PubMed
-
- Van Limbergen J, Russell RK, Drummond HE, et al. . Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology 2008;135:1114–22. doi:10.1053/j.gastro.2008.06.081 - DOI - PubMed
-
- Pigneur B, Seksik P, Viola S, et al. . Natural history of Crohn's disease: comparison between childhood- and adult-onset disease. Inflamm Bowel Dis 2010;16:953–61. doi:10.1002/ibd.21152 - DOI - PubMed
-
- Ruemmele FM, Veres G, Kolho KL, et al. . Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn's disease. J Crohns Colitis 2014;8:1179–207. doi:10.1016/j.crohns.2014.04.005 - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical