Hepatocyte Toll-Like Receptor 5 Promotes Bacterial Clearance and Protects Mice Against High-Fat Diet-Induced Liver Disease
- PMID: 28090564
- PMCID: PMC5042709
- DOI: 10.1016/j.jcmgh.2016.04.007
Hepatocyte Toll-Like Receptor 5 Promotes Bacterial Clearance and Protects Mice Against High-Fat Diet-Induced Liver Disease
Abstract
Background & aims: Innate immune dysfunction can promote chronic inflammatory diseases of the liver. For example, mice lacking the flagellin receptor Toll-like receptor 5 (TLR5) show microbial dysbiosis and predisposition to high-fat diet (HFD)-induced hepatic steatosis. The extent to which hepatocytes play a direct role in detecting bacterial products in general, or flagellin in particular, is poorly understood. In the present study, we investigated the role of hepatocyte TLR5 in recognizing flagellin, policing bacteria, and protecting against liver disease.
Methods: Mice were engineered to lack TLR5 specifically in hepatocytes (TLR5ΔHep) and analyzed relative to sibling controls (TLR5fl/fl). TLR5 messenger RNA levels, responses to exogenous flagellin, elimination of circulating motile bacteria, and susceptibility of liver injury (concanavalin A, carbon tetrachloride, methionine- and choline-deficient diet, and HFD) were measured.
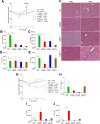
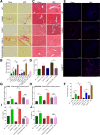
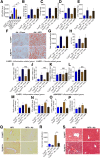
Results: TLR5ΔHep expressed similar levels of TLR5 as TLR5fl/fl in all organs examined, except in the liver, which showed a 90% reduction in TLR5 levels, indicating that hepatocytes accounted for the major portion of TLR5 expression in this organ. TLR5ΔHep showed impairment in responding to purified flagellin and clearing flagellated bacteria from the liver. Although TLR5ΔHep mice did not differ markedly from sibling controls in concanavalin A or carbon tetrachloride-induced liver injury models, they showed exacerbated disease in response to a methionine- and choline-deficient diet and HFD. Such predisposition of TLR5ΔHep to diet-induced liver pathology was associated with increased expression of proinflammatory cytokines, which was dependent on the Nod-like-receptor C4 inflammasome and rescued by microbiota ablation.
Conclusions: Hepatocyte TLR5 plays a critical role in protecting liver against circulating gut bacteria and against diet-induced liver disease.
Keywords: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CCL4, carbon tetrachloride; CFU, colony-forming unit; CXCL, chemokine (C-X-C motif) ligand 1; ConA, concanavalin A; DC, dendritic cell; HFD, high-fat diet; Hep, hepatocyte; Hepatocytes; IEC, intestinal epithelial cell; IL, interleukin; Inflammation; Innate Immunity; KO, knock-out; LPS, lipopolysaccharide; MCD, methionine- and choline-deficient diet; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; NLR, nod-like receptor; NPC, nonparenchymal cell; PBS, phosphate-buffered saline; RT-PCR, reverse-transcription polymerase chain reaction; Steatosis; TLR, Toll-like receptor; TLR5; WT, wild-type; mRNA, messenger RNA.
Figures






References
-
- Hooper L.V., Macpherson A.J. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–169. - PubMed
-
- Chassaing B., Darfeuille-Michaud A. The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1720–1728. - PubMed
-
- Xavier R.J., Podolsky D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. - PubMed
-
- Sartor R.B. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. - PubMed
-
- Cader M.Z., Kaser A. Recent advances in inflammatory bowel disease: mucosal immune cells in intestinal inflammation. Gut. 2013;62:1653–1664. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

