Circadian Forced Desynchrony of the Master Clock Leads to Phenotypic Manifestation of Depression in Rats
- PMID: 28090585
- PMCID: PMC5216685
- DOI: 10.1523/ENEURO.0237-16.2016
Circadian Forced Desynchrony of the Master Clock Leads to Phenotypic Manifestation of Depression in Rats
Abstract
In mammals, a master circadian clock within the suprachiasmatic nucleus (SCN) of the hypothalamus maintains the phase coherence among a wide array of behavioral and physiological circadian rhythms. Affective disorders are typically associated with disruption of this fine-tuned "internal synchronization," but whether this internal misalignment is part of the physiopathology of mood disorders is not clear. To date, depressive-like behavior in animal models has been induced by methods that fail to specifically target the SCN regulation of internal synchronization as the mode to generate depression. In the rat, exposure to a 22-h light-dark cycle (LD22) leads to the uncoupling of two distinct populations of neuronal oscillators within the SCN. This genetically, neurally, and pharmacologically intact animal model represents a unique opportunity to assess the effect of a systematic challenge to the central circadian pacemaker on phenotypic manifestations of mood disorders. We show that LD22 circadian forced desynchrony in rats induces depressive-like phenotypes including anhedonia, sexual dysfunction, and increased immobility in the forced swim test (FST), as well as changes in the levels and turnover rates of monoamines within the prefrontal cortex. Desynchronized rats show increased FST immobility during the dark (active) phase but decreased immobility during the light (rest) phase, suggesting a decrease in the amplitude of the normal daily oscillation in this behavioral manifestation of depression. Our results support the notion that the prolonged internal misalignment of circadian rhythms induced by environmental challenge to the central circadian pacemaker may constitute part of the etiology of depression.
Keywords: behavior; circadian rhythms; depression; emotion; rat.
Figures

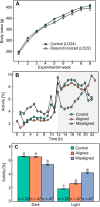




References
-
- Austin M, Whitehead R, Edgar C, Janosky J, Lewis D (2002) Localized decrease in serotonin transporter-immunoreactive axons in the prefrontal cortex of depressed subjects committing suicide. Neuroscience 114:807–815. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
