ultraLM and miniLM: Locator tools for smart tracking of fluorescent cells in correlative light and electron microscopy
- PMID: 28090593
- PMCID: PMC5234702
- DOI: 10.12688/wellcomeopenres.10299.1
ultraLM and miniLM: Locator tools for smart tracking of fluorescent cells in correlative light and electron microscopy
Abstract
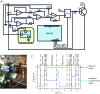
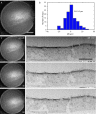
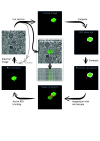
In-resin fluorescence (IRF) protocols preserve fluorescent proteins in resin-embedded cells and tissues for correlative light and electron microscopy, aiding interpretation of macromolecular function within the complex cellular landscape. Dual-contrast IRF samples can be imaged in separate fluorescence and electron microscopes, or in dual-modality integrated microscopes for high resolution correlation of fluorophore to organelle. IRF samples also offer a unique opportunity to automate correlative imaging workflows. Here we present two new locator tools for finding and following fluorescent cells in IRF blocks, enabling future automation of correlative imaging. The ultraLM is a fluorescence microscope that integrates with an ultramicrotome, which enables 'smart collection' of ultrathin sections containing fluorescent cells or tissues for subsequent transmission electron microscopy or array tomography. The miniLM is a fluorescence microscope that integrates with serial block face scanning electron microscopes, which enables 'smart tracking' of fluorescent structures during automated serial electron image acquisition from large cell and tissue volumes.
Keywords: CLEM; Correlative; GFP; In-resin fluorescence; Integrated; Locator tool; Serial block face SEM; Smart tracking; Ultramicrotome; miniLM; ultraLM.
Conflict of interest statement
No competing interests were disclosed.
Figures





References
-
- Brama E, Peddie CJ, Wilkes G, et al. : X, Y displacement in miniLM [Data set]. Zenodo. 2016a. Data Source - DOI
-
- Brama E, Peddie CJ, Wilkes G, et al. : ultraLM and miniLM: Locator tools for smart tracking of fluorescent cells in correlative light and electron microscopy [Data set]. Zenodo. 2016b. Data Source - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

