Repo-Man/PP1 regulates heterochromatin formation in interphase
- PMID: 28091603
- PMCID: PMC5241828
- DOI: 10.1038/ncomms14048
Repo-Man/PP1 regulates heterochromatin formation in interphase
Abstract
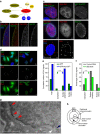
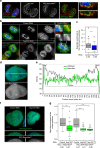
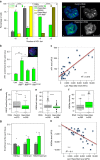
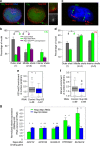
Repo-Man is a protein phosphatase 1 (PP1) targeting subunit that regulates mitotic progression and chromatin remodelling. After mitosis, Repo-Man/PP1 remains associated with chromatin but its function in interphase is not known. Here we show that Repo-Man, via Nup153, is enriched on condensed chromatin at the nuclear periphery and at the edge of the nucleopore basket. Repo-Man/PP1 regulates the formation of heterochromatin, dephosphorylates H3S28 and it is necessary and sufficient for heterochromatin protein 1 binding and H3K27me3 recruitment. Using a novel proteogenomic approach, we show that Repo-Man is enriched at subtelomeric regions together with H2AZ and H3.3 and that depletion of Repo-Man alters the peripheral localization of a subset of these regions and alleviates repression of some polycomb telomeric genes. This study shows a role for a mitotic phosphatase in the regulation of the epigenetic landscape and gene expression in interphase.
Figures


References
-
- Kind J. & van Steensel B. Genome-nuclear lamina interactions and gene regulation. Curr. Opin. Cell Biol. 22, 320–325 (2010). - PubMed
-
- Ye Q. & Worman H. J. Interaction between an integral protein of the nuclear envelope inner membrane and human chromodomain proteins homologous to Drosophila HP1. J. Biol. Chem. 271, 14653–14656 (1996). - PubMed
-
- Bickmore Wendy A. & van Steensel B. Genome architecture: domain organization of interphase chromosomes. Cell 152, 1270–1284 (2013). - PubMed
-
- Fischle W. et al.. Regulation of HP1-chromatin binding by histone H3 methylation and phosphorylation. Nature 438, 1116–1122 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/H017275/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 103139/Z/13/Z/WT_/Wellcome Trust/United Kingdom
- MC_PC_U127527202/MRC_/Medical Research Council/United Kingdom
- MC_U127527202/MRC_/Medical Research Council/United Kingdom
- BB/G011818/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

