Binding of N-methylscopolamine to the extracellular domain of muscarinic acetylcholine receptors
- PMID: 28091608
- PMCID: PMC5238504
- DOI: 10.1038/srep40381
Binding of N-methylscopolamine to the extracellular domain of muscarinic acetylcholine receptors
Abstract
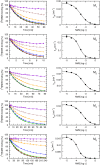
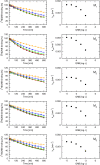
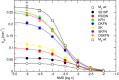
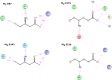
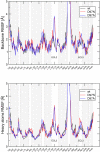
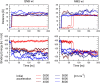
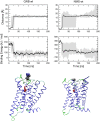
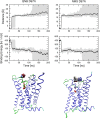
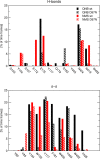
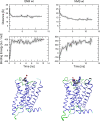
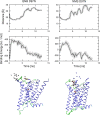
Interaction of orthosteric ligands with extracellular domain was described at several aminergic G protein-coupled receptors, including muscarinic acetylcholine receptors. The orthosteric antagonists quinuclidinyl benzilate (QNB) and N-methylscopolamine (NMS) bind to the binding pocket of the muscarinic acetylcholine receptor formed by transmembrane α-helices. We show that high concentrations of either QNB or NMS slow down dissociation of their radiolabeled species from all five subtypes of muscarinic acetylcholine receptors, suggesting allosteric binding. The affinity of NMS at the allosteric site is in the micromolar range for all receptor subtypes. Using molecular modelling of the M2 receptor we found that E172 and E175 in the second extracellular loop and N419 in the third extracellular loop are involved in allosteric binding of NMS. Mutation of these amino acids to alanine decreased affinity of NMS for the allosteric binding site confirming results of molecular modelling. The allosteric binding site of NMS overlaps with the binding site of some allosteric, ectopic and bitopic ligands. Understanding of interactions of NMS at the allosteric binding site is essential for correct analysis of binding and action of these ligands.
Figures





References
-
- Bonner T. I. The molecular basis of muscarinic receptor diversity. Trends Neurosci 12, 148–51 (1989). - PubMed
-
- Eglen R. M. Overview of Muscarinic Receptor Subtypes In Handb exp pharmacol (eds Fryer A. D., Christopoulos A. & Nathanson N. M.) pp. 3–28, Springer (2012). - PubMed
-
- Jakubík J. et al. Outline of therapeutic interventions with muscarinic receptor-mediated transmission. Physiol Res 63 Suppl 1, S177–89 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

