Carfilzomib, lenalidomide and dexamethasone in patients with heavily pretreated multiple myeloma: A phase 1 study in Japan
- PMID: 28092421
- PMCID: PMC5378280
- DOI: 10.1111/cas.13166
Carfilzomib, lenalidomide and dexamethasone in patients with heavily pretreated multiple myeloma: A phase 1 study in Japan
Abstract
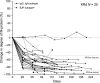
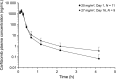
This is the first study in which the carfilzomib, lenalidomide and dexamethasone (KRd) regimen was evaluated in heavily pretreated multiple myeloma. This study is a multicenter, open-label phase 1 study of KRd in Japanese patients with relapsed or refractory multiple myeloma (RRMM) patients. The objectives were to evaluate the safety, tolerability, efficacy and pharmacokinetics of the regimen. Carfilzomib was administrated intravenously over 10 min on days 1, 2, 8, 9, 15 and 16 of a 28-day cycle. In cycle 1, the dosage for days 1 and 2 was 20 mg/m2 , followed by 27 mg/m2 . Lenalidomide and dexamethasone were administered at 25 mg (days 1-21) and 40 mg (days 1, 8, 15 and 22), respectively. Twenty-six patients were enrolled. Patients had received a median of four prior regimens and 88.5% and 61.5% received previous bortezomib and lenalidomide, respectively. High-risk cytogenetics were seen in 53.8% of patients. The overall response rate was 88.5%. A higher rate of hyperglycemia was observed than in a previous carfilzomib monotherapy study, but this was attributed to dexamethasone. Carfilzomib pharmacokinetics were not affected by lenalidomide and dexamethasone. The KRd regimen was well tolerated and showed efficacy in Japanese RRMM patients.
Keywords: Japan; carfilzomib; dexamethasone; lenalidomide; multiple myeloma.
© 2017 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures


References
-
- Mohty B, El‐Cheikh J, Yakoub‐Agha I, Avet‐Loiseau H, Moreau P, Mohty M. Treatment strategies in relapsed and refractory multiple myeloma: a focus on drug sequencing and ‘retreatment’ approaches in the era of novel agents. Leukemia 2012; 26: 73–85. - PubMed
-
- Benboubker L, Dimopoulos MA, Dispenzieri A, et al Lenalidomide and dexamethasone in transplant‐ineligible patients with myeloma. N Engl J Med 2014; 371: 906–17. - PubMed
-
- Stewart AK, Rajkumar SV, Dimopoulos MA, et al Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med 2015; 372: 142–52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

