In vitro effects of hyaluronic acid on human periodontal ligament cells
- PMID: 28093072
- PMCID: PMC5240222
- DOI: 10.1186/s12903-017-0341-1
In vitro effects of hyaluronic acid on human periodontal ligament cells
Abstract
Background: Hyaluronic acid (HA) has been reported to have a positive effect on periodontal wound healing following nonsurgical and surgical therapy. However, to date, a few basic in vitro studies have been reported to investigating the potential of HA on human periodontal ligament (PDL) cell regeneration. Therefore, the aim of this study was to investigate the effect of HA on PDL cell compatibility, proliferation, and differentiation in vitro.
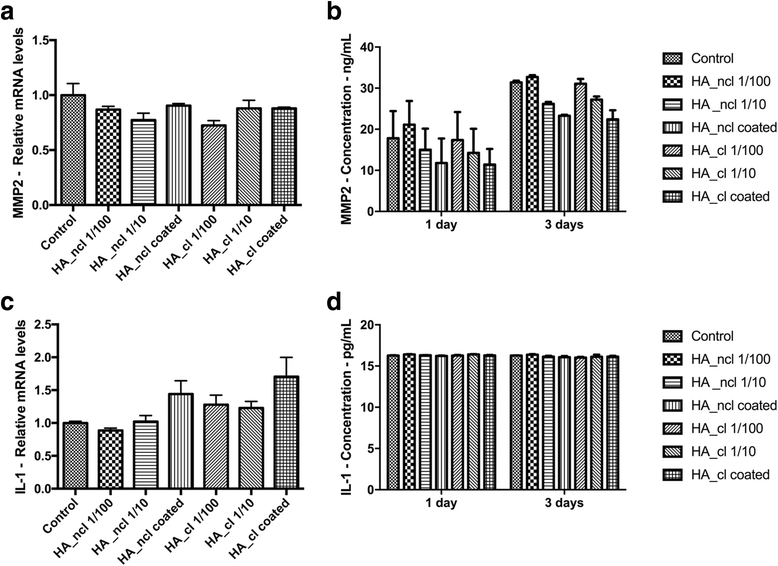
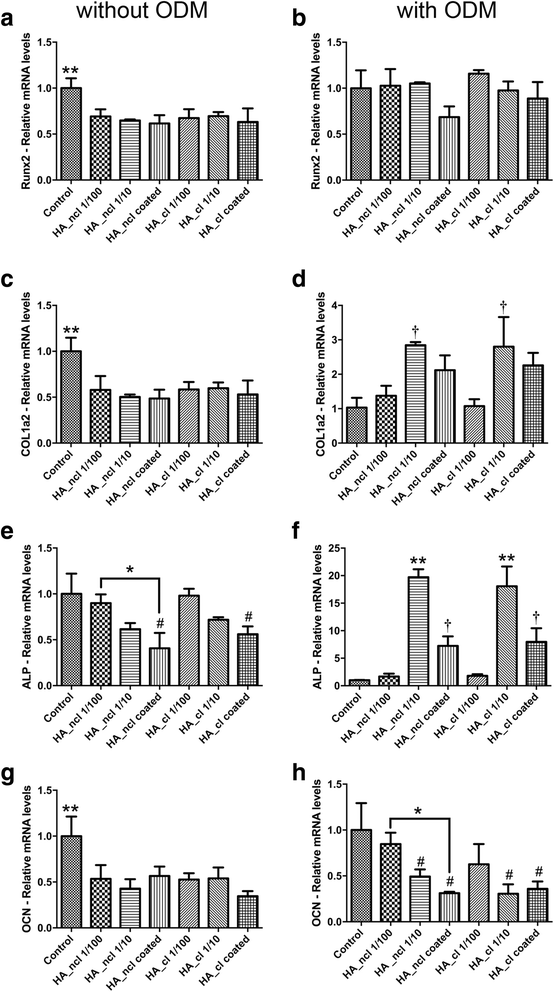
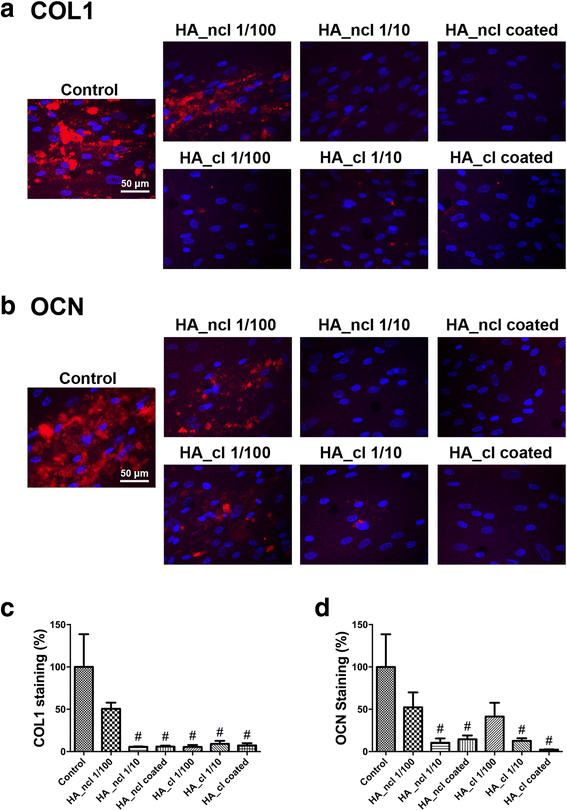
Methods: Either non-cross-linked (HA_ncl) or cross-linked (HA_cl) HA was investigated. Human PDL cells were seeded in 7 conditions as follows (1) Control tissue culture plastic (TCP) (2) dilution of HA_ncl (1:100), (3) dilution of HA_ncl (1:10), 4) HA_ncl directly coated onto TCP, (5) dilution of HA_cl (1:100), 6) dilution of HA_cl (1:10) and (7) HA_cl directly coated onto TCP. Samples were then investigated for cell viability using a live/dead assay, an inflammatory reaction using real-time PCR and ELISA for MMP2, IL-1 and cell proliferation via an MTS assay. Furthermore, the osteogenic potential of PDL cells was assessed by alkaline phosphatase(ALP) activity, collagen1(COL1) and osteocalcin(OCN) immunostaining, alizarin red staining, and real-time PCR for genes encoding Runx2, COL1, ALP, and OCN.
Results: Both HA_ncl and HA_cl showed high PDL cell viability (greater than 90%) irrespective of the culturing conditions. Furthermore, no significant difference in both mRNA and protein levels of proinflammatory cytokines, including MMP2 and IL-1 expression was observed. Both diluted HA_ncl and HA_cl significantly increased cell numbers compared to the controlled TCP samples at 3 and 5 days. HA_ncl and HA_cl in standard cell growth media significantly decreased ALP staining, COL1 immunostaining and down-regulated early osteogenic differentiation, including Runx2, COL1, and OCN mRNA levels when compared to control samples. When osteogenic differentiation medium (ODM) was added, interestingly, the expression of early osteogenic markers increased by demonstrating higher levels of COL1 and ALP expression; especially in HA 1:10 diluted condition. Late stage osteogenic markers remained inhibited.
Conclusions: Both non-cross-linked and cross-linked HA maintained high PDL cell viability, increased proliferation, and early osteogenic differentiation. However, HA was consistently associated with a significant decrease in late osteogenic differentiation of primary human PDL cells. Future in vitro and animal research is necessary to further characterize the effect of HA on periodontal regeneration.
Keywords: Connective tissue regeneration; Hyaluronan; Hyaluronic acid; Periodontal regeneration; Soft tissue regeneration.
Figures




References
-
- Pilloni A, Rimondini L, De Luca M, Bernard GW. Effect of hyaluronan on calcification-nodule formation from human periodontal ligament cell culture. J Appl Biomater Biomech. 2003;1(1):84–90. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

