Inflammasomes and Cancer
- PMID: 28093447
- PMCID: PMC5593081
- DOI: 10.1158/2326-6066.CIR-16-0269
Inflammasomes and Cancer
Abstract
Inflammation affects all stages of tumorigenesis. A key signaling pathway leading to acute and chronic inflammation is through activation of the caspase-1 inflammasome. Inflammasome complexes are assembled on activation of certain nucleotide-binding domain, leucine-rich repeat-containing proteins (NLR), AIM2-like receptors, or pyrin. Of these, NLRP1, NLRP3, NLRC4, NLRP6, and AIM2 influence the pathogenesis of cancer by modulating innate and adaptive immune responses, cell death, proliferation, and/or the gut microbiota. Activation of the inflammasome and IL18 signaling pathways is largely protective in colitis-associated colorectal cancer, whereas excessive inflammation driven by the inflammasome or the IL1 signaling pathways promotes breast cancer, fibrosarcoma, gastric carcinoma, and lung metastasis in a context-dependent manner. The clinical relevance of inflammasomes in multiple forms of cancer highlights their therapeutic promise as molecular targets. In this review, we explore the crossroads between inflammasomes and the development of various tumors and discuss possible therapeutic values in targeting the inflammasome for the prevention and treatment of cancer. Cancer Immunol Res; 5(2); 94-99. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Figures

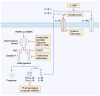
References
-
- de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6(1):24–37. - PubMed
-
- Verma D, Bivik C, Farahani E, Synnerstad I, Fredrikson M, Enerback C, et al. Inflammasome polymorphisms confer susceptibility to sporadic malignant melanoma. Pigment Cell Melanoma Res. 2012;25(4):506–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

