Differential Presynaptic ATP Supply for Basal and High-Demand Transmission
- PMID: 28093477
- PMCID: PMC5320616
- DOI: 10.1523/JNEUROSCI.2712-16.2017
Differential Presynaptic ATP Supply for Basal and High-Demand Transmission
Abstract
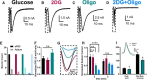
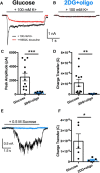
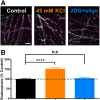
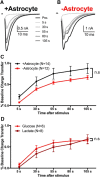
The relative contributions of glycolysis and oxidative phosphorylation to neuronal presynaptic energy demands are unclear. In rat hippocampal neurons, ATP production by either glycolysis or oxidative phosphorylation alone sustained basal evoked synaptic transmission for up to 20 min. However, combined inhibition of both ATP sources abolished evoked transmission. Neither action potential propagation failure nor depressed Ca2+ influx explained loss of evoked synaptic transmission. Rather, inhibition of ATP synthesis caused massive spontaneous vesicle exocytosis, followed by arrested endocytosis, accounting for the disappearance of evoked postsynaptic currents. In contrast to its weak effects on basal transmission, inhibition of oxidative phosphorylation alone depressed recovery from vesicle depletion. Local astrocytic lactate shuttling was not required. Instead, either ambient monocarboxylates or neuronal glycolysis was sufficient to supply requisite substrate. In summary, basal transmission can be sustained by glycolysis, but strong presynaptic demands are met preferentially by oxidative phosphorylation, which can be maintained by bulk but not local monocarboxylates or by neuronal glycolysis.SIGNIFICANCE STATEMENT Neuronal energy levels are critical for proper CNS function, but the relative roles for the two main sources of ATP production, glycolysis and oxidative phosphorylation, in fueling presynaptic function in unclear. Either glycolysis or oxidative phosphorylation can fuel low-frequency synaptic function and inhibiting both underlies loss of synaptic transmission via massive vesicle release and subsequent failure to endocytose lost vesicles. Oxidative phosphorylation, fueled by either glycolysis or endogenously released monocarboxylates, can fuel more metabolically demanding tasks such as vesicle recovery after depletion. Our work demonstrates the flexible nature of fueling presynaptic function to maintain synaptic function.
Keywords: astrocyte; glutamate; glycolysis; neuroenergetics; presynaptic.
Copyright © 2017 the authors 0270-6474/17/371888-12$15.00/0.
Figures




References
-
- Attwell D, Laughlin SB (2001) An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab 21:1133–1145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
