Cytosolic thioredoxin reductase 1 is required for correct disulfide formation in the ER
- PMID: 28093500
- PMCID: PMC5331760
- DOI: 10.15252/embj.201695336
Cytosolic thioredoxin reductase 1 is required for correct disulfide formation in the ER
Abstract
Folding of proteins entering the secretory pathway in mammalian cells frequently requires the insertion of disulfide bonds. Disulfide insertion can result in covalent linkages found in the native structure as well as those that are not, so-called non-native disulfides. The pathways for disulfide formation are well characterized, but our understanding of how non-native disulfides are reduced so that the correct or native disulfides can form is poor. Here, we use a novel assay to demonstrate that the reduction in non-native disulfides requires NADPH as the ultimate electron donor, and a robust cytosolic thioredoxin system, driven by thioredoxin reductase 1 (TrxR1 or TXNRD1). Inhibition of this reductive pathway prevents the correct folding and secretion of proteins that are known to form non-native disulfides during their folding. Hence, we have shown for the first time that mammalian cells have a pathway for transferring reducing equivalents from the cytosol to the ER, which is required to ensure correct disulfide formation in proteins entering the secretory pathway.
Keywords: disulfide formation; endoplasmic reticulum; protein disulfide isomerase; redox homeostasis; thioredoxin reductase.
© 2017 The Authors. Published under the terms of the CC BY 4.0 license.
Figures

- A–D
Cell‐free translation with 35S‐labeling of newly synthesized protein was carried out in the presence of increasing concentrations of G6P as indicated. The transcripts used were (A) c‐VIMP, (B) influenza virus HA, (C) preprolactin, and (D) β1‐integrin. The samples were separated by SDS–PAGE under reducing (lane 1) or non‐reducing (lanes 2–7) conditions. The mobility of the reduced (red) or oxidized (ox) protein is as indicated.

The TrxR1 pathway is driven by NADPH reducing Trx. The reduced Trx can then efficiently reduce disulfides in substrate proteins (P).
The GR pathway is also driven by NADPH resulting in the reduction in glutathione disulfide. The GSH generated can either (i) directly reduce disulfides in substrate proteins or (ii) reduce glutaredoxin (Grx) which then reduces substrate proteins.
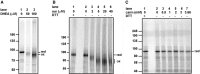
- A–C
Cell‐free translation of β1‐integrin was carried out in the presence of G6P and increasing concentrations of (A) DHEA, (B) auranofin (aur), or (C) carmustine (carm) as indicated. The translation mixture was incubated for 10 min prior to the reaction being initiated by the addition of mRNA and incubation at 30°C. Translation products were separated by SDS–PAGE after prior reduction with DTT (B and C, lane 1) or without reduction (A, lanes 1–3 or B and C, lanes 2–6).

- A–C
Cell‐free translation of influenza virus HA was carried out in the presence of G6P and increasing concentrations of (A) DHEA, (B) auranofin (aur), or (C) carmustine (carm) as indicated. Translation products were separated after prior reduction with DTT (lane 1) or without reduction (A, lanes 2–8 or B and C, lanes 2–6).
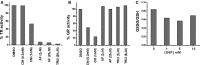
- A, B
The % inhibition of purified human TrxR1 or GR was measured following addition of either carmustine, auranofin, or TRi2 at the indicated concentrations. The experiment was carried out in triplicate with similar results.
- C
The ratio of GSSG/GSH was calculated in a reticulocyte lysate either before or after addition of G6P at the indicated concentration. The ratios are calculated from the average values for [GSH] or [GSSG] from three determinations.

The oxidation status of added Trx1 was assayed through immunoblotting following a 60‐min incubation in a cell‐free translation system in the absence or presence of G6P with and without auranofin (aur) as indicated. Following incubation, Trx1 was isolated, reduced, and any free thiols released were modified with AMS which retards electrophoretic mobility. Hence, the slower migrating band of the doublet is Trx1 that was oxidized in the original sample. The position of the reduced (red) or oxidized (ox) protein is as indicated.
Reticulocyte lysate was treated with or without 5 μM purified ChaC1 for 60 min prior to measuring the GSH concentration. The results are the average from three separate determinations ± SD.
Cell‐free translation of β1 integrin was carried out in the presence or absence of G6P as indicated and in normal (lanes 1–3) or GSH‐depleted (lanes 4 and 5) reticulocyte lysate. Samples were separated with (lane 1) or without (lanes 2–5) prior reduction with DTT.

Translation of β1‐integrin was carried out in the presence of SP cells and the absence or presence of G6P as indicated. Samples were separated with or without prior reduction with DTT as indicated. Auranofin (aur; 20 μM) was either included in the reticulocyte lysate (L) during translation (lane 7) or the SP cells (C) were pre‐treated with auranofin (lane 8).
Translations of β1‐integrin carried out in the presence of SP cells (lanes 1 and 2) were subjected to immunoisolation with the conformation‐specific antibody 9EG7 (lanes 3 and 4). Translations were carried out in the absence and presence of G6P as indicated and samples separated under non‐reducing conditions.
As in (A), but carmustine (carm; 1 mM) was used instead of auranofin.

- A–C
HT1080 cells were metabolically radiolabeled with 35S for 30 min and then chased in the absence of radiolabel for up to 60 min as indicated in the presence of vehicle (DMSO) (A), 5 μM auranofin (B), or 5 μM TRi2 (C). Radiolabeled LDLr was immunoisolated with anti‐LDLr and samples separated before or after reduction as indicated. The position of the Golgi (G) and ER forms migrating under non‐reducing conditions are as indicated.
- D, E
HT1080 cells transfected with V5‐tagged α1‐antitrypsin were radiolabeled and cells chased in the absence (D) or presence (E) of auranofin as in (A–C) and radiolabeled products immunoisolated with a V5‐antibody.
References
-
- Arner ES, Holmgren A (2000) Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem 267: 6102–6109 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

