A model of type 2 diabetes in the guinea pig using sequential diet-induced glucose intolerance and streptozotocin treatment
- PMID: 28093504
- PMCID: PMC5312002
- DOI: 10.1242/dmm.025593
A model of type 2 diabetes in the guinea pig using sequential diet-induced glucose intolerance and streptozotocin treatment
Abstract
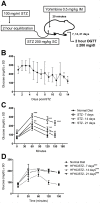
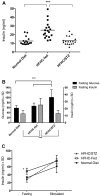
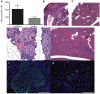
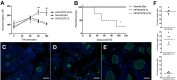
Type 2 diabetes is a leading cause of morbidity and mortality among noncommunicable diseases, and additional animal models that more closely replicate the pathogenesis of human type 2 diabetes are needed. The goal of this study was to develop a model of type 2 diabetes in guinea pigs, in which diet-induced glucose intolerance precedes β-cell cytotoxicity, two processes that are crucial to the development of human type 2 diabetes. Guinea pigs developed impaired glucose tolerance after 8 weeks of feeding on a high-fat, high-carbohydrate diet, as determined by oral glucose challenge. Diet-induced glucose intolerance was accompanied by β-cell hyperplasia, compensatory hyperinsulinemia, and dyslipidemia with hepatocellular steatosis. Streptozotocin (STZ) treatment alone was ineffective at inducing diabetic hyperglycemia in guinea pigs, which failed to develop sustained glucose intolerance or fasting hyperglycemia and returned to euglycemia within 21 days after treatment. However, when high-fat, high-carbohydrate diet-fed guinea pigs were treated with STZ, glucose intolerance and fasting hyperglycemia persisted beyond 21 days post-STZ treatment. Guinea pigs with diet-induced glucose intolerance subsequently treated with STZ demonstrated an insulin-secretory capacity consistent with insulin-independent diabetes. This insulin-independent state was confirmed by response to oral antihyperglycemic drugs, metformin and glipizide, which resolved glucose intolerance and extended survival compared with guinea pigs with uncontrolled diabetes. In this study, we have developed a model of sequential glucose intolerance and β-cell loss, through high-fat, high-carbohydrate diet and extensive optimization of STZ treatment in the guinea pig, which closely resembles human type 2 diabetes. This model will prove useful in the study of insulin-independent diabetes pathogenesis with or without comorbidities, where the guinea pig serves as a relevant model species.
Keywords: Animal model; Glucose intolerance; Guinea pig; Insulin-independent diabetes; Streptozotocin; Type 2 diabetes.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures


Similar articles
-
Association of insulin resistance with hyperglycemia in streptozotocin-diabetic pigs: effects of metformin at isoenergetic feeding in a type 2-like diabetic pig model.Metabolism. 2006 Jul;55(7):960-71. doi: 10.1016/j.metabol.2006.03.004. Metabolism. 2006. PMID: 16784971
-
Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening.Pharmacol Res. 2005 Oct;52(4):313-20. doi: 10.1016/j.phrs.2005.05.004. Pharmacol Res. 2005. PMID: 15979893
-
Reduction of oxidative stress by a new low-molecular-weight antioxidant improves metabolic alterations in a nonobese mouse diabetes model.Pancreas. 2007 Nov;35(4):e10-7. doi: 10.1097/mpa.0b013e318150e4f2. Pancreas. 2007. PMID: 18090226
-
Type II diabetes mellitus.Adv Intern Med. 1998;43:449-500. Adv Intern Med. 1998. PMID: 9506190 Review.
-
Goals of treatment for type 2 diabetes: beta-cell preservation for glycemic control.Diabetes Care. 2009 Nov;32 Suppl 2(Suppl 2):S178-83. doi: 10.2337/dc09-S306. Diabetes Care. 2009. PMID: 19875548 Free PMC article. Review. No abstract available.
Cited by
-
One Size Fits All? Not in In Vivo Modeling of Tuberculosis Chemotherapeutics.Front Cell Infect Microbiol. 2021 Mar 16;11:613149. doi: 10.3389/fcimb.2021.613149. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33796474 Free PMC article. Review.
-
The Potential Therapeutic Effect of Orexin-Treated versus Orexin-Untreated Adipose Tissue-Derived Mesenchymal Stem Cell Therapy on Insulin Resistance in Type 2 Diabetic Rats.J Diabetes Res. 2022 Jan 17;2022:9832212. doi: 10.1155/2022/9832212. eCollection 2022. J Diabetes Res. 2022. PMID: 35083338 Free PMC article.
-
Electrical Features of the Diabetic Myocardium. Arrhythmic and Cardiovascular Safety Considerations in Diabetes.Front Pharmacol. 2021 Jul 8;12:687256. doi: 10.3389/fphar.2021.687256. eCollection 2021. Front Pharmacol. 2021. PMID: 34305599 Free PMC article. Review.
-
Molecular and Electrophysiological Role of Diabetes-Associated Circulating Inflammatory Factors in Cardiac Arrhythmia Remodeling in a Metabolic-Induced Model of Type 2 Diabetic Rat.Int J Mol Sci. 2021 Jun 25;22(13):6827. doi: 10.3390/ijms22136827. Int J Mol Sci. 2021. PMID: 34202017 Free PMC article.
-
Advances in protein subunit vaccines against tuberculosis.Front Immunol. 2023 Aug 15;14:1238586. doi: 10.3389/fimmu.2023.1238586. eCollection 2023. Front Immunol. 2023. PMID: 37654500 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

