Hebbian plasticity requires compensatory processes on multiple timescales
- PMID: 28093557
- PMCID: PMC5247595
- DOI: 10.1098/rstb.2016.0259
Hebbian plasticity requires compensatory processes on multiple timescales
Abstract
We review a body of theoretical and experimental research on Hebbian and homeostatic plasticity, starting from a puzzling observation: while homeostasis of synapses found in experiments is a slow compensatory process, most mathematical models of synaptic plasticity use rapid compensatory processes (RCPs). Even worse, with the slow homeostatic plasticity reported in experiments, simulations of existing plasticity models cannot maintain network stability unless further control mechanisms are implemented. To solve this paradox, we suggest that in addition to slow forms of homeostatic plasticity there are RCPs which stabilize synaptic plasticity on short timescales. These rapid processes may include heterosynaptic depression triggered by episodes of high postsynaptic firing rate. While slower forms of homeostatic plasticity are not sufficient to stabilize Hebbian plasticity, they are important for fine-tuning neural circuits. Taken together we suggest that learning and memory rely on an intricate interplay of diverse plasticity mechanisms on different timescales which jointly ensure stability and plasticity of neural circuits.This article is part of the themed issue 'Integrating Hebbian and homeostatic plasticity'.
Keywords: Hebbian plasticity; heterosynaptic plasticity; homeostasis; metaplasticity; rapid compensatory processes; synaptic scaling.
© 2017 The Authors.
Figures


 (dashed). (b) Same as in (a), but with τ = 2η−1. Model: we simulated
(dashed). (b) Same as in (a), but with τ = 2η−1. Model: we simulated  ,
,  and y = wx with x ≡ 1.
and y = wx with x ≡ 1.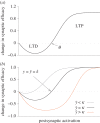
 is larger than the target value κ, θ(t) shifts to higher values. Likewise, θ(t) shifts to lower values when
is larger than the target value κ, θ(t) shifts to higher values. Likewise, θ(t) shifts to lower values when  is too low. For
is too low. For  changes in synaptic efficacy are zero.
changes in synaptic efficacy are zero.
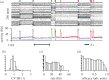
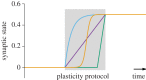
 . With a slow homeostatic mechanism, a neuron can track input relatively accurately. (c) Same as b, but for a rapid compensatory process (τslow = 50τfast) which drives the activity quickly to the target value. If the timescale of feedback is comparable to that of the stimulus, negative feedback interferes with the neuron's ability to track the stimulus. (d) RCPs enforcing an allowed range (limits indicated by dotted lines). Even though the neuronal activity does not capture all the diversity of the input, it does capture some of it. Here, we modelled the RCPs as the following nonlinear extension of the above model:
. With a slow homeostatic mechanism, a neuron can track input relatively accurately. (c) Same as b, but for a rapid compensatory process (τslow = 50τfast) which drives the activity quickly to the target value. If the timescale of feedback is comparable to that of the stimulus, negative feedback interferes with the neuron's ability to track the stimulus. (d) RCPs enforcing an allowed range (limits indicated by dotted lines). Even though the neuronal activity does not capture all the diversity of the input, it does capture some of it. Here, we modelled the RCPs as the following nonlinear extension of the above model:  with f(x) = x for
with f(x) = x for  and f(x) = 0 otherwise.
and f(x) = 0 otherwise.

References
-
- Hebb DO. 1949. The organization of behavior: a neuropsychological theory. New York, NY: John Wiley & Sons.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
