Collagen hydrogel scaffold promotes mesenchymal stem cell and endothelial cell coculture for bone tissue engineering
- PMID: 28093887
- PMCID: PMC5328802
- DOI: 10.1002/jbm.a.36008
Collagen hydrogel scaffold promotes mesenchymal stem cell and endothelial cell coculture for bone tissue engineering
Abstract
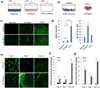
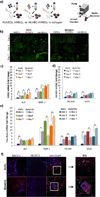
The generation of functional, vascularized tissues is a key challenge for the field of tissue engineering. Before clinical implantations of such tissue engineered bone constructs can succeed, tactics to promote neovascularization need to be strengthened. We have previously demonstrated that the tubular perfusion system (TPS) bioreactor is an effective culturing method to augment osteogenic differentiation and maintain viability of human mesenchymal stem cells (hMSC). Here, we devised a strategy to address the need for a functional microvasculature by designing an in vitro coculture system that simultaneously cultures osteogenic differentiating hMSCs with endothelial cells (ECs). We utilized the TPS bioreactor as a dynamic coculture environment, which we hypothesize will encourage prevascularization of endothelial cells and early formation of bone tissue and could aid in anastomosis of the graft with the host vasculature after patient implantation. To evaluate the effect of different natural scaffolds for this coculture system, the cells were encapsulated in alginate and/or collagen hydrogel scaffolds. We discovered the necessity of cell-to-cell proximity between the two cell types as well as preference for the natural cell binding capabilities of hydrogels like collagen. We discovered increased osteogenic and angiogenic potential as seen by amplified gene and protein expression of ALP, BMP-2, VEGF, and PECAM. The TPS bioreactor further augmented these expressions, indicating a synergistic effect between coculture and applied shear stress. The development of this dynamic coculture platform for the prevascularization of engineered bone, emphasizing the importance of the construct microenvironments and will advance the clinical use of tissue engineered constructs. © 2017 Wiley Periodicals, Inc. J Biomed Mater Res Part A: 105A: 1123-1131, 2017.
Keywords: TPS bioreactor; bone regeneration; coculture; endothelial cells; hydrogel scaffold; mesenchymal stem cells.
© 2017 Wiley Periodicals, Inc.
Figures

References
-
- Fiedler T, Belova IV, Murch GE, Poologasundarampillai G, Jones JR, Roether JA, et al. A comparative study of oxygen diffusion in tissue engineering scaffolds. J. Mater. Sci. Mater. Med. 2014;25(11):2573. - PubMed
-
- Unger RE, Ghanaati S, Orth C, Sartoris A, Barbeck M, Halstenberg S, et al. The rapid anastomosis between prevascularized networks on silk fibroin scaffolds generated in vitro with cocultures of human microvascular endothelial and osteoblast cells and the host vasculature. Biomaterials. 2010;31(27):6959. - PubMed
-
- Ma J, van den Beucken JJJP, Yang F, Both SK, Cui F-Z, Pan J, et al. Coculture of osteoblasts and endothelial cells: optimization of culture medium and cell ratio. Tissue Eng. Part C. Methods. 2011;17(3):349. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

