Optimizing isothiocyanate formation during enzymatic glucosinolate breakdown by adjusting pH value, temperature and dilution in Brassica vegetables and Arabidopsis thaliana
- PMID: 28094342
- PMCID: PMC5240131
- DOI: 10.1038/srep40807
Optimizing isothiocyanate formation during enzymatic glucosinolate breakdown by adjusting pH value, temperature and dilution in Brassica vegetables and Arabidopsis thaliana
Abstract
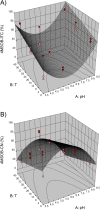
Consumption of glucosinolate-rich Brassicales vegetables is associated with a decreased risk of cancer with enzymatic hydrolysis of glucosinolates playing a key role. However, formation of health-promoting isothiocyanates is inhibited by the epithiospecifier protein in favour of nitriles and epithionitriles. Domestic processing conditions, such as changes in pH value, temperature or dilution, might also affect isothiocyanate formation. Therefore, the influences of these three factors were evaluated in accessions of Brassica rapa, Brassica oleracea, and Arabidopsis thaliana. Mathematical modelling was performed to determine optimal isothiocyanate formation conditions and to obtain knowledge on the kinetics of the reactions. At 22 °C and endogenous plant pH, nearly all investigated plants formed nitriles and epithionitriles instead of health-promoting isothiocyanates. Response surface models, however, clearly demonstrated that upon change in pH to domestic acidic (pH 4) or basic pH values (pH 8), isothiocyanate formation considerably increases. While temperature also affects this process, the pH value has the greatest impact. Further, a kinetic model showed that isothiocyanate formation strongly increases due to dilution. Finally, the results show that isothiocyanate intake can be strongly increased by optimizing the conditions of preparation of Brassicales vegetables.
Figures




References
-
- Kissen R., Rossiter J. & Bones A. The ‘mustard oil bomb’: not so easy to assemble?! Localization, expression and distribution of the components of the myrosinase enzyme system. Phytochem. Rev. 8, 69–86, doi: 10.1007/s11101-008-9109-1 (2009). - DOI
-
- Shofran B. G., Purrington S. T., Breidt F. & Fleming H. P. Antimicrobial properties of sinigrin and its hydrolysis products. J. Food Sci. 63, 621–624, doi: 10.1111/j.1365-2621.1998.tb15798.x (1998). - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

