Artificial Cells: Synthetic Compartments with Life-like Functionality and Adaptivity
- PMID: 28094501
- PMCID: PMC5397886
- DOI: 10.1021/acs.accounts.6b00512
Artificial Cells: Synthetic Compartments with Life-like Functionality and Adaptivity
Abstract
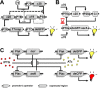
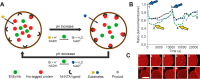
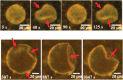
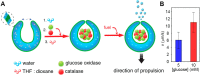
Cells are highly advanced microreactors that form the basis of all life. Their fascinating complexity has inspired scientists to create analogs from synthetic and natural components using a bottom-up approach. The ultimate goal here is to assemble a fully man-made cell that displays functionality and adaptivity as advanced as that found in nature, which will not only provide insight into the fundamental processes in natural cells but also pave the way for new applications of such artificial cells. In this Account, we highlight our recent work and that of others on the construction of artificial cells. First, we will introduce the key features that characterize a living system; next, we will discuss how these have been imitated in artificial cells. First, compartmentalization is crucial to separate the inner chemical milieu from the external environment. Current state-of-the-art artificial cells comprise subcompartments to mimic the hierarchical architecture of eukaryotic cells and tissue. Furthermore, synthetic gene circuits have been used to encode genetic information that creates complex behavior like pulses or feedback. Additionally, artificial cells have to reproduce to maintain a population. Controlled growth and fission of synthetic compartments have been demonstrated, but the extensive regulation of cell division in nature is still unmatched. Here, we also point out important challenges the field needs to overcome to realize its full potential. As artificial cells integrate increasing orders of functionality, maintaining a supporting metabolism that can regenerate key metabolites becomes crucial. Furthermore, life does not operate in isolation. Natural cells constantly sense their environment, exchange (chemical) signals, and can move toward a chemoattractant. Here, we specifically explore recent efforts to reproduce such adaptivity in artificial cells. For instance, synthetic compartments have been produced that can recruit proteins to the membrane upon an external stimulus or modulate their membrane composition and permeability to control their interaction with the environment. A next step would be the communication of artificial cells with either bacteria or another artificial cell. Indeed, examples of such primitive chemical signaling are presented. Finally, motility is important for many organisms and has, therefore, also been pursued in synthetic systems. Synthetic compartments that were designed to move in a directed, controlled manner have been assembled, and directed movement toward a chemical attractant is among one of the most life-like directions currently under research. Although the bottom-up construction of an artificial cell that can be truly considered "alive" is still an ambitious goal, the recent work discussed in this Account shows that this is an active field with contributions from diverse disciplines like materials chemistry and biochemistry. Notably, research during the past decade has already provided valuable insights into complex synthetic systems with life-like properties. In the future, artificial cells are thought to contribute to an increased understanding of processes in natural cells and provide opportunities to create smart, autonomous, cell-like materials.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Gánti T.; Griesemer J.; Szathmáry E.. The Principles of Life, 1st ed.; Oxford University Press: Oxford, 2003.
-
- Sokolova E.; Spruijt E.; Hansen M. M. K.; Dubuc E.; Groen J.; Chokkalingam V.; Piruska A.; Heus H. A.; Huck W. T. S. Enhanced Transcription Rates in Membrane-Free Protocells Formed by Coacervation of Cell Lysate. Proc. Natl. Acad. Sci. U. S. A. 2013, 110 (29), 11692–11697. 10.1073/pnas.1222321110. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

