Noninvasive Targeted Transcranial Neuromodulation via Focused Ultrasound Gated Drug Release from Nanoemulsions
- PMID: 28094959
- PMCID: PMC5362146
- DOI: 10.1021/acs.nanolett.6b03517
Noninvasive Targeted Transcranial Neuromodulation via Focused Ultrasound Gated Drug Release from Nanoemulsions
Abstract
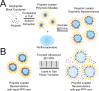
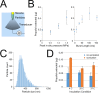
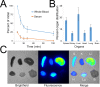
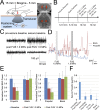
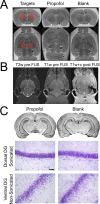
Targeted, noninvasive neuromodulation of the brain of an otherwise awake subject could revolutionize both basic and clinical neuroscience. Toward this goal, we have developed nanoparticles that allow noninvasive uncaging of a neuromodulatory drug, in this case the small molecule anesthetic propofol, upon the application of focused ultrasound. These nanoparticles are composed of biodegradable and biocompatible constituents and are activated using sonication parameters that are readily achievable by current clinical transcranial focused ultrasound systems. These particles are potent enough that their activation can silence seizures in an acute rat seizure model. Notably, there is no evidence of brain parenchymal damage or blood-brain barrier opening with their use. Further development of these particles promises noninvasive, focal, and image-guided clinical neuromodulation along a variety of pharmacological axes.
Keywords: Neuromodulation; focused ultrasound; gated drug release; nanoparticles.
Conflict of interest statement
The authors declare the following competing financial interest(s): Dr. Nicholas Ellen has worked in the past as a paid consultant for FUS Instruments, Inc.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

