Phase I First-in-Human Study of Venetoclax in Patients With Relapsed or Refractory Non-Hodgkin Lymphoma
- PMID: 28095146
- PMCID: PMC5455685
- DOI: 10.1200/JCO.2016.70.4320
Phase I First-in-Human Study of Venetoclax in Patients With Relapsed or Refractory Non-Hodgkin Lymphoma
Abstract
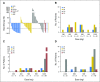
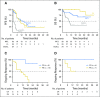
Purpose B-cell leukemia/lymphoma-2 (BCL-2) overexpression is common in many non-Hodgkin lymphoma (NHL) subtypes. A phase I trial in patients with NHL was conducted to determine safety, pharmacokinetics, and efficacy of venetoclax, a selective, potent, orally bioavailable BCL-2 inhibitor. Patients and Methods A total of 106 patients with relapsed or refractory NHL received venetoclax once daily until progressive disease or unacceptable toxicity at target doses from 200 to 1,200 mg in dose-escalation and safety expansion cohorts. Treatment commenced with a 3-week dose ramp-up period for most patients in dose-escalation cohorts and for all patients in safety expansion. Results NHL subtypes included mantle cell lymphoma (MCL; n = 28), follicular lymphoma (FL; n = 29), diffuse large B-cell lymphoma (DLBCL; n = 34), DLBCL arising from chronic lymphocytic leukemia (Richter transformation; n = 7), Waldenström macroglobulinemia (n = 4), and marginal zone lymphoma (n = 3). Venetoclax was generally well tolerated. Clinical tumor lysis syndrome was not observed, whereas laboratory tumor lysis syndrome was documented in three patients. Treatment-emergent adverse events were reported in 103 patients (97%), a majority of which were grade 1 to 2 in severity. Grade 3 to 4 events were reported in 59 patients (56%), and the most common were hematologic, including anemia (15%), neutropenia (11%), and thrombocytopenia (9%). Overall response rate was 44% (MCL, 75%; FL, 38%; DLBCL, 18%). Estimated median progression-free survival was 6 months (MCL, 14 months; FL, 11 months; DLBCL, 1 month). Conclusion Selective targeting of BCL-2 with venetoclax was well tolerated, and single-agent activity varied among NHL subtypes. We determined 1,200 mg to be the recommended single-agent dose for future studies in FL and DLBCL, with 800 mg being sufficient to consistently achieve durable response in MCL. Additional investigations including combination therapy to augment response rates and durability are ongoing.
Figures


References
-
- McDonnell TJ, Deane N, Platt FM, et al. Bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989;57:79–88. - PubMed
-
- Tsujimoto Y, Finger LR, Yunis J, et al. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984;226:1097–1099. - PubMed
-
- Huang JZ, Sanger WG, Greiner TC, et al. The t(14;18) defines a unique subset of diffuse large B-cell lymphoma with a germinal center B-cell gene expression profile. Blood. 2002;99:2285–2290. - PubMed
-
- Bentz M, Plesch A, Bullinger L, et al. t(11;14)-positive mantle cell lymphomas exhibit complex karyotypes and share similarities with B-cell chronic lymphocytic leukemia. Genes Chromosomes Cancer. 2000;27:285–294. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

