A randomised phase II trial and feasibility study of palliative chemotherapy in frail or elderly patients with advanced gastroesophageal cancer (321GO)
- PMID: 28095397
- PMCID: PMC5318975
- DOI: 10.1038/bjc.2016.442
A randomised phase II trial and feasibility study of palliative chemotherapy in frail or elderly patients with advanced gastroesophageal cancer (321GO)
Abstract
Background: Elderly patients are commonly under-represented in cancer clinical trials. The 321GO was undertaken in preparation for a definitive phase three trial assessing different chemotherapy regimens in a frail and/or elderly population with advanced gastroesophageal (GO) cancer.
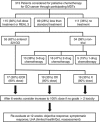
Methods: Patients with advanced GO cancer considered unfit for conventional dose chemotherapy were randomly assigned in a 1 : 1 : 1 ratio to: epirubicin, oxaliplatin and capecitabine (EOX); oxaliplatin and capecitabine (OX); and capecitabine alone (X) (all 80% of full dose and unblinded). The primary end point was patient recruitment over an 18-month period. A registration study recorded treatment choice for all patients with advanced GO cancer at trial centres.
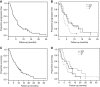
Results: A total of 313 patients were considered for palliative chemotherapy for GO cancer over the 18-month period: 115 received full dose treatment, 89 less than standard treatment or entered 321GO and 111 no treatment. Within 321GO, 55 patients were randomly assigned (19 to OX and X; 17 to EOX). Progression-free survival (PFS) for all patients was 4.4 months and by arm 5.4, 5.6 and 3.0 months for EOX, OX and X, respectively. The number of patients with a good overall treatment utility (OTU), a novel patient-centred endpoint, at 12 weeks was 3 (18%), 6 (32%) and 1 (6%) for EOX, OX and X, respectively. At 6 weeks, 22 patients (41%) had experienced a non-haematologic toxicity ⩾grade 3, most commonly lethargy or diarrhoea. The OTU was prognostic for overall survival in patients alive at week 12 (logrank test P=0.0001).
Conclusions: It is feasible to recruit elderly and/or frail patients with advanced GO cancer to a randomised clinical trial. The OX is the preferred regimen for further study. Overall treatment utility shows promise as a comparator between treatment regimens for feasibility and randomised trials in the elderly and/or frail GO cancer population.
Conflict of interest statement
Roche provided an unconditional grant but had no influence over the design or publication of this study. MTS has received travel, accommodation and departmental research funding (unconnected with 321GO) from Roche. PSH was formerly employed by the University of Leeds on a research grant from Roche as a trial administrator for an unconnected project. SRL has received travel and accommodation funding from Roche (unconnected with 321GO). MTS, HM, MJ, and HH are employed by the University of Leeds, the study sponsor.
Figures
References
-
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JCJM Kaasa S, Klee M, Osoba D, Razavi D, Rofe PB, Schraub S, Sneeuw K, Sullivan M, Takeda F (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85: 365–376. - PubMed
-
- Alderson D, Langley RE, Nankivell MG Blazeby JM, Griffin M, Crellin A, Grabsch HI, Okines AFC, Goldstein C, Falk S, Thompson J, Krysztopik R, Coxon FY, Pritchard S, Langer R, Stenning SP, Cunningham D (2015) Neoadjuvant chemotherapy for resectable oesophageal and junctional adenocarcinoma: results from the UK Medical Research Council randomised OEO5 trial (ISRCTN 01852072). J Clin Oncol 33:(suppl; abstr 4002).
-
- Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40: 373–383. - PubMed
-
- Cunningham D, Okines AF, Ashley S (2010) Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 362: 858–859. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

