Computational Analysis of Residue Interaction Networks and Coevolutionary Relationships in the Hsp70 Chaperones: A Community-Hopping Model of Allosteric Regulation and Communication
- PMID: 28095400
- PMCID: PMC5240922
- DOI: 10.1371/journal.pcbi.1005299
Computational Analysis of Residue Interaction Networks and Coevolutionary Relationships in the Hsp70 Chaperones: A Community-Hopping Model of Allosteric Regulation and Communication
Abstract
Allosteric interactions in the Hsp70 proteins are linked with their regulatory mechanisms and cellular functions. Despite significant progress in structural and functional characterization of the Hsp70 proteins fundamental questions concerning modularity of the allosteric interaction networks and hierarchy of signaling pathways in the Hsp70 chaperones remained largely unexplored and poorly understood. In this work, we proposed an integrated computational strategy that combined atomistic and coarse-grained simulations with coevolutionary analysis and network modeling of the residue interactions. A novel aspect of this work is the incorporation of dynamic residue correlations and coevolutionary residue dependencies in the construction of allosteric interaction networks and signaling pathways. We found that functional sites involved in allosteric regulation of Hsp70 may be characterized by structural stability, proximity to global hinge centers and local structural environment that is enriched by highly coevolving flexible residues. These specific characteristics may be necessary for regulation of allosteric structural transitions and could distinguish regulatory sites from nonfunctional conserved residues. The observed confluence of dynamics correlations and coevolutionary residue couplings with global networking features may determine modular organization of allosteric interactions and dictate localization of key mediating sites. Community analysis of the residue interaction networks revealed that concerted rearrangements of local interacting modules at the inter-domain interface may be responsible for global structural changes and a population shift in the DnaK chaperone. The inter-domain communities in the Hsp70 structures harbor the majority of regulatory residues involved in allosteric signaling, suggesting that these sites could be integral to the network organization and coordination of structural changes. Using a network-based formalism of allostery, we introduced a community-hopping model of allosteric communication. Atomistic reconstruction of signaling pathways in the DnaK structures captured a direction-specific mechanism and molecular details of signal transmission that are fully consistent with the mutagenesis experiments. The results of our study reconciled structural and functional experiments from a network-centric perspective by showing that global properties of the residue interaction networks and coevolutionary signatures may be linked with specificity and diversity of allosteric regulation mechanisms.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


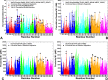



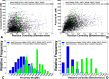


Similar articles
-
Dancing through Life: Molecular Dynamics Simulations and Network-Centric Modeling of Allosteric Mechanisms in Hsp70 and Hsp110 Chaperone Proteins.PLoS One. 2015 Nov 30;10(11):e0143752. doi: 10.1371/journal.pone.0143752. eCollection 2015. PLoS One. 2015. PMID: 26619280 Free PMC article.
-
Exploring Molecular Mechanisms of Paradoxical Activation in the BRAF Kinase Dimers: Atomistic Simulations of Conformational Dynamics and Modeling of Allosteric Communication Networks and Signaling Pathways.PLoS One. 2016 Nov 18;11(11):e0166583. doi: 10.1371/journal.pone.0166583. eCollection 2016. PLoS One. 2016. PMID: 27861609 Free PMC article.
-
Probing Allosteric Inhibition Mechanisms of the Hsp70 Chaperone Proteins Using Molecular Dynamics Simulations and Analysis of the Residue Interaction Networks.J Chem Inf Model. 2016 Aug 22;56(8):1490-517. doi: 10.1021/acs.jcim.5b00755. Epub 2016 Aug 1. J Chem Inf Model. 2016. PMID: 27447295
-
Intra-molecular pathways of allosteric control in Hsp70s.Philos Trans R Soc Lond B Biol Sci. 2018 Jun 19;373(1749):20170183. doi: 10.1098/rstb.2017.0183. Philos Trans R Soc Lond B Biol Sci. 2018. PMID: 29735737 Free PMC article. Review.
-
Interrogating Regulatory Mechanisms in Signaling Proteins by Allosteric Inhibitors and Activators: A Dynamic View Through the Lens of Residue Interaction Networks.Adv Exp Med Biol. 2019;1163:187-223. doi: 10.1007/978-981-13-8719-7_9. Adv Exp Med Biol. 2019. PMID: 31707705 Review.
Cited by
-
Ensemble-Based Binding Free Energy Profiling and Network Analysis of the KRAS Interactions with DARPin Proteins Targeting Distinct Binding Sites: Revealing Molecular Determinants and Universal Architecture of Regulatory Hotspots and Allosteric Binding.Biomolecules. 2025 Jun 5;15(6):819. doi: 10.3390/biom15060819. Biomolecules. 2025. PMID: 40563459 Free PMC article.
-
Machine learning and protein allostery.Trends Biochem Sci. 2023 Apr;48(4):375-390. doi: 10.1016/j.tibs.2022.12.001. Epub 2022 Dec 21. Trends Biochem Sci. 2023. PMID: 36564251 Free PMC article. Review.
-
A folding nucleus and minimal ATP binding domain of Hsp70 identified by single-molecule force spectroscopy.Proc Natl Acad Sci U S A. 2018 May 1;115(18):4666-4671. doi: 10.1073/pnas.1716899115. Epub 2018 Apr 18. Proc Natl Acad Sci U S A. 2018. PMID: 29669923 Free PMC article.
-
Probing conformational landscapes and mechanisms of allosteric communication in the functional states of the ABL kinase domain using multiscale simulations and network-based mutational profiling of allosteric residue potentials.J Chem Phys. 2022 Dec 28;157(24):245101. doi: 10.1063/5.0133826. J Chem Phys. 2022. PMID: 36586979 Free PMC article.
-
The Hsp70 interdomain linker is a dynamic switch that enables allosteric communication between two structured domains.J Biol Chem. 2017 Sep 8;292(36):14765-14774. doi: 10.1074/jbc.M117.789313. Epub 2017 Jul 28. J Biol Chem. 2017. PMID: 28754691 Free PMC article.
References
-
- Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92: 351–366. - PubMed
-
- Mayer MP, Brehmer D, Gässler CS, Bukau B. Hsp70 chaperone machines. Adv Protein Chem. 2001;59: 1–44. - PubMed
-
- Meimaridou E, Gooljar SB, Chapple JP. From hatching to dispatching: the multiple cellular roles of the Hsp70 molecular chaperone machinery. J Mol Endocrinol. 2009;42: 1–9. - PubMed
-
- Mayer MP. Gymnastics of molecular chaperones. Mol Cell. 2010;39: 321–331. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

