Cytoplasmic Localization of HTLV-1 HBZ Protein: A Biomarker of HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis (HAM/TSP)
- PMID: 28095504
- PMCID: PMC5271414
- DOI: 10.1371/journal.pntd.0005285
Cytoplasmic Localization of HTLV-1 HBZ Protein: A Biomarker of HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis (HAM/TSP)
Abstract
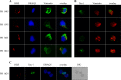
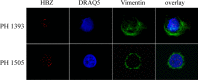
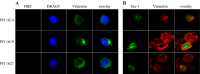
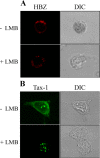
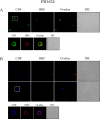
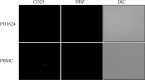
HTLV-1 is the causative agent of a severe form of adult T cell leukemia/Lymphoma (ATL), and of a chronic progressive neuromyelopathy designated HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP). Two important HTLV-1-encoded proteins, Tax-1 and HBZ, play crucial roles in the generation and maintenance of the oncogenic process. Less information is instead available on the molecular and cellular mechanisms leading to HAM/TSP. More importantly, no single specific biomarker has been described that unambiguously define the status of HAM/TSP. Here we report for the first time the finding that HBZ, described until now as an exclusive nuclear protein both in chronically infected and in ATL cells, is instead exclusively localized in the cytoplasm of peripheral blood mononuclear cells (PBMC) from patients suffering of HAM/TSP. Interestingly, at the single cell level, HBZ and Tax-1 proteins are never found co-expressed in the same cell, suggesting the existence of mechanisms of expression uncoupling of these two important HTLV-1 viral products in HAM/TSP patients. Cells expressing cytoplasmic HBZ were almost exclusively found in the CD4+ T cell compartment that was not, at least in a representative HAM/TSP patient, expressing the CD25 marker. Less than 1 percent CD8+ T cells were fond positive for HBZ, while B cells and NK cells were found negative for HBZ in HAM/TSP patients. Our results identify the cytoplasmic localization of HBZ in HAM/TSP patient as a possible biomarker of this rather neglected tropical disease, and raise important hypotheses on the role of HBZ in the pathogenesis of the neuromyelopathy associated to HTLV-1 infection.
Conflict of interest statement
The authors have declared that no competing interests exist
Figures

References
-
- Gessain A, Barin F, Vernant JC, Gout O, Maurs L, Calender A, et al. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;2:407–10. - PubMed
-
- Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, et al. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;1:1031–2. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

