Effectiveness of medication review: a systematic review and meta-analysis of randomized controlled trials
- PMID: 28095780
- PMCID: PMC5240219
- DOI: 10.1186/s12875-016-0577-x
Effectiveness of medication review: a systematic review and meta-analysis of randomized controlled trials
Abstract
Background: Medication review is often recommended to optimize medication use. In clinical practice it is mostly operationalized as an intervention without co-interventions during a short term intervention period. However, most systematic reviews also included co-interventions and prolonged medication optimization interventions. Furthermore, most systematic reviews focused on specific patient groups (e.g. polypharmacy, elderly, hospitalized) and/or on specific outcome measures (e.g. hospital admissions and mortality). Therefore, the objective of this study is to assess the effectiveness of medication review as an isolated short-term intervention, irrespective of the patient population and the outcome measures used.
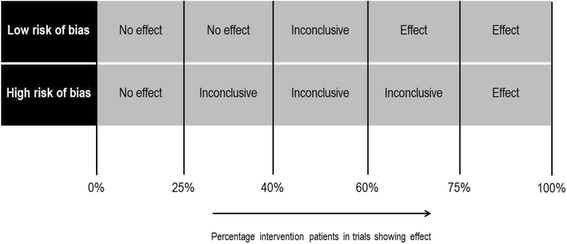
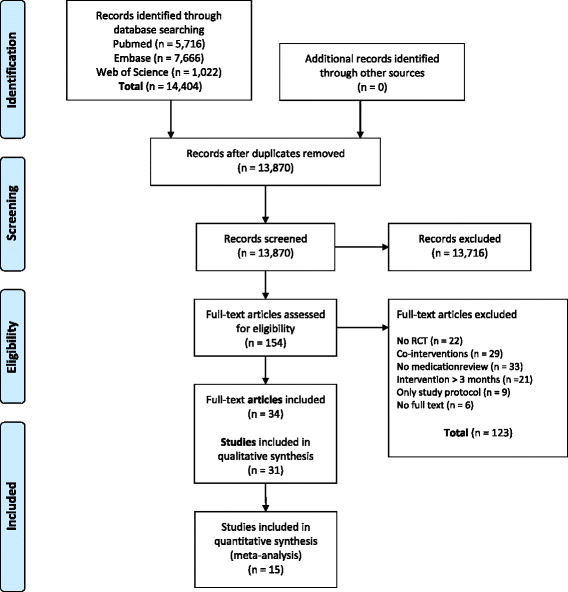
Methods: A literature search was performed in MEDLINE, EMBASE and Web of Science from their inception through September 2015. Randomized controlled trials (RCTs) with medication review as isolated short term intervention (<3 months) were included. There were no restrictions with regard to patient characteristics and outcome measures. One reviewer extracted and a second checked data. The risk of bias of studies was evaluated independently by two reviewers. A best evidence synthesis was conducted for every outcome measure used in more than one trial. In case of binary variables a meta-analysis was performed in addition to the best evidence synthesis, to quantify the effect.
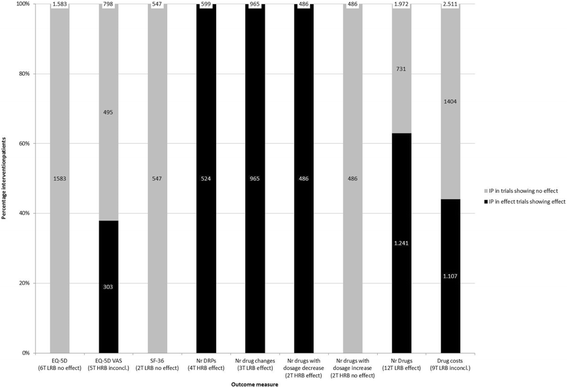
Results: Thirty-one RCTs were included in this systematic review (55% low risk of bias). A best evidence synthesis was conducted for 22 outcome measures. No effect of medication review was found on clinical outcomes (mortality, hospital admissions/healthcare use, the number of patients falling, physical and cognitive functioning), except a decrease in the number of falls per patient. However, in a sensitivity analysis using a more stringent threshold for risk of bias, the conclusion for the effect on the number of falls changed to inconclusive. Furthermore no effect was found on quality of life and evidence was inconclusive about the effect on economical outcome measures. However, an effect was found on most drug-related problems: medication review resulted in a decrease in the number of drug-related problems, more changes in medication, more drugs with dosage decrease and a greater decrease or smaller increase of the number of drugs.
Conclusions: An isolated medication review during a short term intervention period has an effect on most drug-related outcomes, minimal effect on clinical outcomes and no effect on quality of life. No conclusion can be drawn about the effect on economical outcome measures. Therefore, it should be considered to stop performing cross-sectional medication reviews as standard care.
Keywords: Clinical outcomes; Drug utilization review[Mesh]; Drug-related outcomes; Economical outcomes; Medication review; Meta-analysis[Publication Type]; Pharmaceutical services[Mesh]; Quality of life; Review[Publication Type].
Figures

References
-
- Medicines optimisation: the safe and effective use of medicines to enable the best possible outcomes. http://www.nice.org.uk/guidance/NG5/chapter/1-recommendations#/medicatio.... Accessed 1 Feb 2016. - PubMed
-
- Multidisciplinary guideline polypharmacy in the elderly 2012 (NHG). https://www.nhg.org/sites/default/files/content/nhg_org/uploads/polyfarm.... Accessed 1 Feb 2016.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

