Providing lipid-based nutrient supplement during pregnancy does not reduce the risk of maternal P falciparum parasitaemia and reproductive tract infections: a randomised controlled trial
- PMID: 28095801
- PMCID: PMC5240436
- DOI: 10.1186/s12884-016-1215-2
Providing lipid-based nutrient supplement during pregnancy does not reduce the risk of maternal P falciparum parasitaemia and reproductive tract infections: a randomised controlled trial
Abstract
Background: Maternal infections are associated with maternal and foetal adverse outcomes. Nutrient supplementation during pregnancy may reduce the occurrence of infections by improving maternal immunity. We aimed to investigate the impact of small-quantity lipid-based nutrient supplement (SQ-LNS) on the occurrence of Plasmodium falciparum parasitaemia during pregnancy and trichomoniasis, vaginal candidiasis and urinary tract infection (UTI) after delivery.
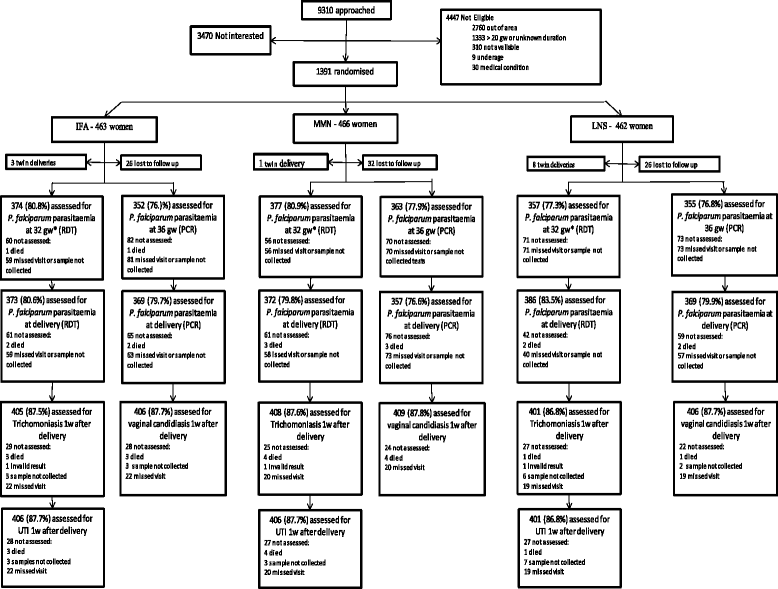
Methods: Pregnant Malawian women enrolled in the iLiNS-DYAD trial receiving daily supplementation with SQ-LNS, multiple micronutrients (MMN) or iron & folic acid (IFA) from <20 gestation weeks (gw) were assessed for P. falciparum parasitaemia at 32 gw using rapid diagnostic testing (RDT), at 36 gw using polymerase chain reaction (PCR) and at delivery using both RDT and PCR; and at one week after delivery for trichomoniasis and vaginal candidiasis using wet mount microscopy and for UTI using urine dipstick analysis. The prevalence of each infection by intervention group was estimated at the prescribed time points and the global null hypothesis was tested using logistic regression. Adjusted analyses were performed using preselected covariates.
Results: The prevalence of P. falciparum parasitaemia was 10.7% at 32 gw, 9% at 36 gw, and 8.3% by RDT and 20.2% by PCR at delivery. After delivery the prevalence of trichomoniasis was 10.5%, vaginal candidiasis was 0.5%, and UTI was 3.1%. There were no differences between intervention groups in the prevalence of any of the infections.
Conclusion: In this population, SQ-LNS did not influence the occurrence of maternal P. falciparum parasitaemia, trichomoniasis, vaginal candidiasis or UTI.
Trial registration: Identifier: NCT01239693 (10 November 2010).
Keywords: Micronutrients; Plasmodium falciparum; Pregnancy; Reproductive tract infections; Urinary tract infection.
References
-
- Steketee RW, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg. 2001;64(1–2 Suppl):28–35. - PubMed
-
- Steketee RW, Wirima JJ, Hightower AW, Slutsker L, Heymann DL, Breman JG. The effect of malaria and malaria prevention in pregnancy on offspring birthweight, prematurity, and intrauterine growth retardation in rural Malawi. Am J Trop Med Hyg. 1996;55(1 Suppl):33–41. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical