Glycogen storage disease type Ia mice with less than 2% of normal hepatic glucose-6-phosphatase-α activity restored are at risk of developing hepatic tumors
- PMID: 28096054
- PMCID: PMC5346453
- DOI: 10.1016/j.ymgme.2017.01.003
Glycogen storage disease type Ia mice with less than 2% of normal hepatic glucose-6-phosphatase-α activity restored are at risk of developing hepatic tumors
Abstract
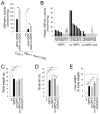
Glycogen storage disease type Ia (GSD-Ia), characterized by impaired glucose homeostasis and chronic risk of hepatocellular adenoma (HCA) and carcinoma (HCC), is caused by a deficiency in glucose-6-phosphatase-α (G6Pase-α or G6PC). We have previously shown that G6pc-/- mice receiving gene transfer mediated by rAAV-G6PC, a recombinant adeno-associated virus (rAAV) vector expressing G6Pase-α, and expressing 3-63% of normal hepatic G6Pase-α activity maintain glucose homeostasis and do not develop HCA/HCC. However, the threshold of hepatic G6Pase-α activity required to prevent tumor formation remained unknown. In this study, we constructed rAAV-co-G6PC, a rAAV vector expressing a codon-optimized (co) G6Pase-α and showed that rAAV-co-G6PC was more efficacious than rAAV-G6PC in directing hepatic G6Pase-α expression. Over an 88-week study, we showed that both rAAV-G6PC- and rAAV-co-G6PC-treated G6pc-/- mice expressing 3-33% of normal hepatic G6Pase-α activity (AAV mice) maintained glucose homeostasis, lacked HCA/HCC, and were protected against age-related obesity and insulin resistance. Of the eleven rAAV-G6PC/rAAV-co-G6PC-treated G6pc-/- mice harboring 0.9-2.4% of normal hepatic G6Pase-α activity (AAV-low mice), 3 expressing 0.9-1.3% of normal hepatic G6Pase-α activity developed HCA/HCC, while 8 did not (AAV-low-NT). Finally, we showed that the AAV-low-NT mice exhibited a phenotype indistinguishable from that of AAV mice expressing ≥3% of normal hepatic G6Pase-α activity. The results establish the threshold of hepatic G6Pase-α activity required to prevent HCA/HCC and show that GSD-Ia mice harboring <2% of normal hepatic G6Pase-α activity are at risk of tumor development.
Keywords: Gene therapy; Hepatocellular adenoma; Hepatocellular carcinoma; Recombinant adeno-associated virus vector.
Published by Elsevier Inc.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures
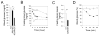
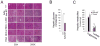
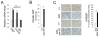
References
-
- Chou JY, Matern D, Mansfield BC, Chen YT. Type I glycogen storage diseases: disorders of the glucose-6-phosphatase complex. Curr Mol Med. 2002;2:121–143. - PubMed
-
- Pan CJ, Lei KJ, Annabi B, Hemrika W, Chou JY. Transmembrane topology of glucose-6-phosphatase. J Biol Chem. 1998;273:6144–6148. - PubMed
-
- Greene HL, Slonim AE, O’Neill JA, Jr, Burr IM. Continuous nocturnal intragastric feeding for management of type 1 glycogen-storage disease. N Engl J Med. 1976;294:423–425. - PubMed
-
- Chen YT, Cornblath M, Sidbury JB. Cornstarch therapy in type I glycogen storage disease. N Engl J Med. 1984;310:171–175. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

