Platelets activate a pathogenic response to blood-stage Plasmodium infection but not a protective immune response
- PMID: 28096086
- PMCID: PMC5364340
- DOI: 10.1182/blood-2016-08-733519
Platelets activate a pathogenic response to blood-stage Plasmodium infection but not a protective immune response
Abstract
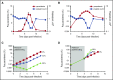
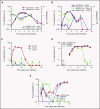
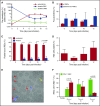
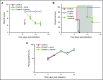
Clinical studies indicate that thrombocytopenia correlates with the development of severe falciparum malaria, suggesting that platelets either contribute to control of parasite replication, possibly as innate parasite killer cells or function in eliciting pathogenesis. Removal of platelets by anti-CD41 mAb treatment, platelet inhibition by aspirin, and adoptive transfer of wild-type (WT) platelets to CD40-KO mice, which do not control parasite replication, resulted in similar parasitemia compared with control mice. Human platelets at a physiologic ratio of 1 platelet to 9 red blood cells (RBCs) did not inhibit the in vitro development or replication of blood-stage Plasmodium falciparum The percentage of Plasmodium-infected (iRBCs) with bound platelets during the ascending parasitemia in Plasmodium chabaudi- and Plasmodium berghei-infected mice and the 48-hour in vitro cycle of P falciparum was <10%. P chabaudi and P berghei iRBCs with apoptotic parasites (TdT+) exhibited minimal platelet binding (<5%), which was similar to nonapoptotic iRBCs. These findings collectively indicate platelets do not kill bloodstage Plasmodium at physiologically relevant effector-to-target ratios. P chabaudi primary and secondary parasitemia was similar in mice depleted of platelets by mAb-injection just before infection, indicating that activation of the protective immune response does not require platelets. In contrast to the lack of an effect on parasite replication, adoptive transfer of WT platelets to CD40-KO mice, which are resistant to experimental cerebral malaria, partially restored experimental cerebral malaria mortality and symptoms in CD40-KO recipients, indicating platelets elicit pathogenesis and platelet CD40 is a key molecule.
© 2017 by The American Society of Hematology.
Figures


Comment in
-
Platelets: killers of parasites or patients?Blood. 2017 Mar 23;129(12):1571-1572. doi: 10.1182/blood-2017-01-764621. Blood. 2017. PMID: 28336729 No abstract available.
References
-
- van der Heyde HC, Nolan J, Combes V, Gramaglia I, Grau GE. A unified hypothesis for the genesis of cerebral malaria: sequestration, inflammation and hemostasis leading to microcirculatory dysfunction. Trends Parasitol. 2006;22(11):503-508. - PubMed
-
- McMorran BJ, Wieczorski L, Drysdale KE, et al. Platelet factor 4 and Duffy antigen required for platelet killing of Plasmodium falciparum. Science. 2012;338(6112):1348-1351. - PubMed
-
- McMorran BJ, Marshall VM, de Graaf C, et al. Platelets kill intraerythrocytic malarial parasites and mediate survival to infection. Science. 2009;323(5915):797-800. - PubMed
-
- Peyron F, Polack B, Lamotte D, Kolodie L, Ambroise-Thomas P. Plasmodium falciparum growth inhibition by human platelets in vitro. Parasitology. 1989;99(Pt 3):317-322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

