Allogeneic hematopoietic stem cell transplantation for MDS and CMML: recommendations from an international expert panel
- PMID: 28096091
- PMCID: PMC5524528
- DOI: 10.1182/blood-2016-06-724500
Allogeneic hematopoietic stem cell transplantation for MDS and CMML: recommendations from an international expert panel
Abstract
An international expert panel, active within the European Society for Blood and Marrow Transplantation, European LeukemiaNet, Blood and Marrow Transplant Clinical Trial Group, and the International Myelodysplastic Syndromes Foundation developed recommendations for allogeneic hematopoietic stem cell transplantation (HSCT) in myelodysplastic syndromes (MDS) and chronic myelomonocytic leukemia (CMML). Disease risks scored according to the revised International Prognostic Scoring System (IPSS-R) and presence of comorbidity graded according to the HCT Comorbidity Index (HCT-CI) were recognized as relevant clinical variables for HSCT eligibility. Fit patients with higher-risk IPSS-R and those with lower-risk IPSS-R with poor-risk genetic features, profound cytopenias, and high transfusion burden are candidates for HSCT. Patients with a very high MDS transplantation risk score, based on combination of advanced age, high HCT-CI, very poor-risk cytogenetic and molecular features, and high IPSS-R score have a low chance of cure with standard HSCT and consideration should be given to treating these patients in investigational studies. Cytoreductive therapy prior to HSCT is advised for patients with ≥10% bone marrow myeloblasts. Evidence from prospective randomized clinical trials does not provide support for specific recommendations on the optimal high intensity conditioning regimen. For patients with contraindications to high-intensity preparative regimens, reduced intensity conditioning should be considered. Optimal timing of HSCT requires careful evaluation of the available effective nontransplant strategies. Prophylactic donor lymphocyte infusion (DLI) strategies are recommended in patients at high risk of relapse after HSCT. Immune modulation by DLI strategies or second HSCT is advised if relapse occurs beyond 6 months after HSCT.
© 2017 by The American Society of Hematology.
Figures
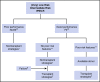
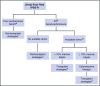
References
-
- Kröger N. From nuclear to a global family: more donors for MDS. Blood. 2013;122(11):1848-1850. - PubMed
-
- Oosterveld M, Suciu S, Muus P, et al. Specific scoring systems to predict survival of patients with high-risk myelodysplastic syndrome (MDS) and de novo acute myeloid leukemia (AML) after intensive antileukemic treatment based on results of the EORTC-GIMEMA AML-10 and intergroup CRIANT studies. Ann Hematol. 2015;94(1):23-34. - PubMed
-
- Fenaux P, Giagounidis A, Selleslag D, et al. ; MDS-004 Lenalidomide del5q Study Group. A randomized phase 3 study of lenalidomide versus placebo in RBC transfusion-dependent patients with low-/intermediate-1-risk myelodysplastic syndromes with del5q. Blood. 2011;118(14):3765-3776. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

