Gain-of-Function Mutants of the Cytokinin Receptors AHK2 and AHK3 Regulate Plant Organ Size, Flowering Time and Plant Longevity
- PMID: 28096190
- PMCID: PMC5338655
- DOI: 10.1104/pp.16.01903
Gain-of-Function Mutants of the Cytokinin Receptors AHK2 and AHK3 Regulate Plant Organ Size, Flowering Time and Plant Longevity
Abstract
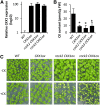
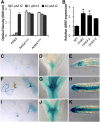
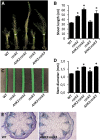
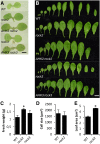
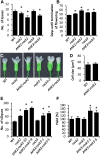
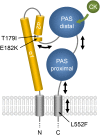
The phytohormone cytokinin is a regulator of numerous processes in plants. In Arabidopsis (Arabidopsis thaliana), the cytokinin signal is perceived by three membrane-located receptors named ARABIDOPSIS HISTIDINE KINASE2 (AHK2), AHK3, and AHK4/CRE1. How the signal is transmitted across the membrane is an entirely unknown process. The three receptors have been shown to operate mostly in a redundant fashion, and very few specific roles have been attributed to single receptors. Using a forward genetic approach, we isolated constitutively active gain-of-function variants of the AHK2 and AHK3 genes, named repressor of cytokinin deficiency2 (rock2) and rock3, respectively. It is hypothesized that the structural changes caused by these mutations in the sensory and adjacent transmembrane domains emulate the structural changes caused by cytokinin binding, resulting in domain motion propagating the signal across the membrane. Detailed analysis of lines carrying rock2 and rock3 alleles revealed how plants respond to locally enhanced cytokinin signaling. Early flowering time, a prolonged reproductive growth phase, and, thereby, increased seed yield suggest that cytokinin regulates various aspects of reproductive growth. In particular, it counteracts the global proliferative arrest, a correlative inhibition of maternal growth by seeds, an as yet unknown activity of the hormone.
© 2017 American Society of Plant Biologists. All Rights Reserved.
Figures




References
-
- Ainley WM, McNeil KJ, Hill JW, Lingle WL, Simpson RB, Brenner ML, Nagao RT, Key JL (1993) Regulatable endogenous production of cytokinins up to ‘toxic’ levels in transgenic plants and plant tissues. Plant Mol Biol 22: 13–23 - PubMed
-
- Ashikari M, Sakakibara H, Lin S, Yamamoto T, Takashi T, Nishimura A, Angeles ER, Qian Q, Kitano H, Matsuoka M (2005) Cytokinin oxidase regulates rice grain production. Science 309: 741–745 - PubMed
-
- Besnard-Wibaut C. (1981) Effectiveness of gibberellins and 6-benzyladenine on flowering of Arabidopsis thaliana. Physiol Plant 53: 205–212
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

