Endothelial cell regulation of salivary gland epithelial patterning
- PMID: 28096213
- PMCID: PMC5394760
- DOI: 10.1242/dev.142497
Endothelial cell regulation of salivary gland epithelial patterning
Abstract
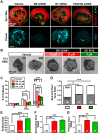
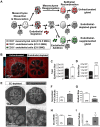
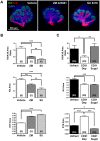
Perfusion-independent regulation of epithelial pattern formation by the vasculature during organ development and regeneration is of considerable interest for application in restoring organ function. During murine submandibular salivary gland development, the vasculature co-develops with the epithelium during branching morphogenesis; however, it is not known whether the vasculature has instructive effects on the epithelium. Using pharmacological inhibitors and siRNA knockdown in embryonic organ explants, we determined that VEGFR2-dependent signaling is required for salivary gland epithelial patterning. To test directly for a requirement for endothelial cells in instructive epithelial patterning, we developed a novel ex vivo cell fractionation/reconstitution assay. Immuno-depletion of CD31+ endothelial cells in this assay confirmed a requirement for endothelial cells in epithelial patterning of the gland. Depletion of endothelial cells or inhibition of VEGFR2 signaling in organ explants caused an aberrant increase in cells expressing the ductal proteins K19 and K7, with a reduction in Kit+ progenitor cells in the endbuds of reconstituted glands. Addition of exogenous endothelial cells to reconstituted glands restored epithelial patterning, as did supplementation with the endothelial cell-regulated mesenchymal factors IGFBP2 and IGFBP3. Our results demonstrate that endothelial cells promote expansion of Kit+ progenitor cells and suppress premature ductal differentiation in early developing embryonic submandibular salivary gland buds.
Keywords: Ductal differentiation; Endothelial cell; Endothelial cells; Epithelial patterning; Mouse; Progenitor cell; Salivary gland development.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures


References
-
- Besnard V., Corroyer S., Trugnan G., Chadelat K., Nabeyrat E., Cazals V. and Clement A. (2001). Distinct patterns of insulin-like growth factor binding protein (IGFBP)-2 and IGFBP-3 expression in oxidant exposed lung epithelial cells. Biochim. Biophys. Acta 1538, 47-58. 10.1016/S0167-4889(00)00136-1 - DOI - PubMed
-
- Binkert C., Margot J. B., Landwehr J., Heinrich G. and Schwander J. (1992). Structure of the human insulin-like growth factor binding protein-2 gene. Mol. Endocrinol. 6, 826-836. - PubMed
-
- Bourner M. J., Busby W. H., Siegel N. R., Krivi G. G., McCusker R. H. and Clemmons D. R. (1992). Cloning and sequence determination of bovine insulin-like growth factor binding protein-2 (IGFBP-2) - comparison of its structural and functional properties with IGFBP-1. J. Cell. Biochem. 48, 215-226. 10.1002/jcb.240480212 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

